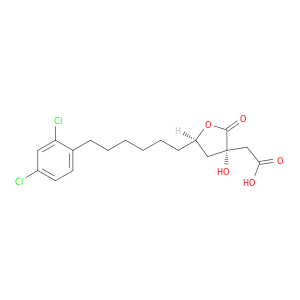
(3R,5S)-rel-5-[6-(2,4-Dichlorophenyl)hexyl]tetrahydro-3-hydroxy-2-oxo-3-furanaceticacid
| Title | Journal |
|---|---|
| Highly efficient and concise synthesis of both antipodes of SB204900, clausenamide, neoclausenamide, homoclausenamide and zeta-clausenamide. Implication of biosynthetic pathways of Clausena alkaloids. | Organic & biomolecular chemistry 20090621 |
| The biology and chemistry of hyperlipidemia. | Bioorganic & medicinal chemistry 20070715 |
| ATP citrate lyase inhibition can suppress tumor cell growth. | Cancer cell 20051001 |
| The role of adenosine triphosphate citrate lyase in the metabolism of acetyl coenzyme a and function of blood platelets in diabetes mellitus. | Metabolism: clinical and experimental 20040101 |