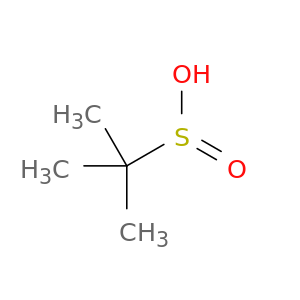
2-methylpropane-2-sulfinic acid
| Title | Journal |
|---|---|
| Sequential ortho-lithiations; the sulfoxide group as a relay to enable meta-substitution. | Organic & biomolecular chemistry 20080407 |
| Palladium-catalyzed enantioselective 1,3-rearrangement of racemic allylic sulfinates: asymmetric synthesis of allylic sulfones and kinetic resolution of an allylic sulfinate. | The Journal of organic chemistry 20040416 |
| Highly selective palladium catalyzed kinetic resolution and enantioselective substitution of racemic allylic carbonates with sulfur nucleophiles: asymmetric synthesis of allylic sulfides, allylic sulfones, and allylic alcohols. | Chemistry (Weinheim an der Bergstrasse, Germany) 20030905 |