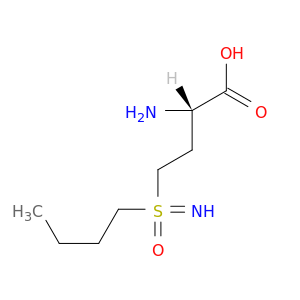
L-Buthionine-(s,r)-sulfoximine
| Title | Journal |
|---|---|
| Collateral sensitivity of multidrug-resistant cells to the orphan drug tiopronin. | Journal of medicinal chemistry 20110728 |
| Extracellular redox modulation by regulatory T cells. | Nature chemical biology 20091001 |
| Genetic mapping of targets mediating differential chemical phenotypes in Plasmodium falciparum. | Nature chemical biology 20091001 |
| Protein S-guanylation by the biological signal 8-nitroguanosine 3',5'-cyclic monophosphate. | Nature chemical biology 20071101 |
| Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. | Proceedings of the National Academy of Sciences of the United States of America 20070724 |
| Sustained ER Ca2+ depletion suppresses protein synthesis and induces activation-enhanced cell death in mast cells. | The Journal of biological chemistry 20020419 |