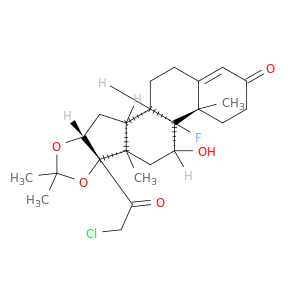
(1S,2S,4R,8S,9S,11S,12R,13S)-8-(2-chloroacetyl)-12-fluoro-11-hydroxy-6,6,9,13-tetramethyl-5,7-dioxapentacyclo[10.8.0.0^{2,9}.0^{4,8}.0^{13,18}]icos-17-en-16-one
| Title | Journal |
|---|---|
| Demonstration of the biphasic release of 0.1% halcinonide cream. | Journal of drugs in dermatology : JDD 20150101 |
| Clobetasol and Halcinonide Act as Smoothened Agonists to Promote Myelin Gene Expression and RxRγ Receptor Activation. | PloS one 20150101 |
| Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps). | PLoS computational biology 20111201 |
| Profiling of a prescription drug library for potential renal drug-drug interactions mediated by the organic cation transporter 2. | Journal of medicinal chemistry 20110714 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Allergic contact dermatitis from dichlorobenzyl alcohol in a patient with multiple contact allergies. | Contact dermatitis 20090501 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Dead Sea extract sold under-the-counter. | The British journal of dermatology 20030701 |
| Tropical application of halcinonide cream reduces the severity and incidence of intraperitoneal adhesions in a rat model. | American journal of surgery 20020701 |
| Clobetasol propionate cream versus halcinonide cream in psoriasis. | International journal of dermatology 19860601 |
| A comparative study of amcinonide and halcinonide in the treatment of eczematous dermatitis. | Cutis 19840801 |
| A controlled comparison of amcinonide cream 0.1 percent and halcinonide cream 0.1 percent in the treatment of eczematous dermatitis. | Cutis 19811001 |