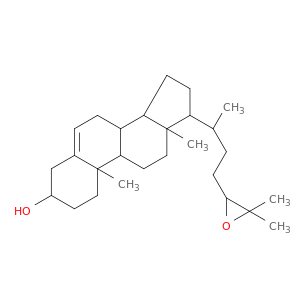
(8S,9S,10R,13R,14S,17R)-17-((R)-4-((S)-3,3-Dimethyloxiran-2-yl)butan-2-yl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol
| Title | Journal |
|---|---|
| Upregulation of hydroxysteroid sulfotransferase 2B1b promotes hepatic oval cell proliferation by modulating oxysterol-induced LXR activation in a mouse model of liver injury. | Archives of toxicology 20170101 |
| Brain endogenous liver X receptor ligands selectively promote midbrain neurogenesis. | Nature chemical biology 20130201 |
| The endogenous regulator 24(S),25-epoxycholesterol inhibits cholesterol synthesis at DHCR24 (Seladin-1). | Biochimica et biophysica acta 20120901 |
| Liver X receptors agonists impede hepatitis C virus infection in an Idol-dependent manner. | Antiviral research 20120901 |
| [Research progress of liver X receptor agonists]. | Yao xue xue bao = Acta pharmaceutica Sinica 20120401 |
| Mast cell death induced by 24(S),25-epoxycholesterol. | Experimental cell research 20101115 |
| Low-density lipoprotein and oxysterols suppress the transcription of CTP: Phosphoethanolamine cytidylyltransferase in vitro. | Biochimica et biophysica acta 20100401 |
| Enhanced steatosis by nuclear receptor ligands: a study in cultured human hepatocytes and hepatoma cells with a characterized nuclear receptor expression profile. | Chemico-biological interactions 20100330 |
| LXR-activating oxysterols induce the expression of inflammatory markers in endothelial cells through LXR-independent mechanisms. | Atherosclerosis 20091101 |
| Ligand, receptor, and cell type-dependent regulation of ABCA1 and ABCG1 mRNA in prostate cancer epithelial cells. | Molecular cancer therapeutics 20090701 |
| Oxysterols: Sources, cellular storage and metabolism, and new insights into their roles in cholesterol homeostasis. | Molecular aspects of medicine 20090601 |
| 24(S),25-epoxycholesterol: a messenger for cholesterol homeostasis. | The international journal of biochemistry & cell biology 20090401 |
| Epoxycholesterol impairs cholesteryl ester hydrolysis in macrophage foam cells, resulting in decreased cholesterol efflux. | Arteriosclerosis, thrombosis, and vascular biology 20080601 |
| Ligand specificity and evolution of liver X receptors. | The Journal of steroid biochemistry and molecular biology 20080501 |
| Combination of virtual screening and high throughput gene profiling for identification of novel liver X receptor modulators. | Journal of medicinal chemistry 20080410 |
| Endogenous 24(S),25-epoxycholesterol fine-tunes acute control of cellular cholesterol homeostasis. | The Journal of biological chemistry 20080111 |
| [Synthesis of sterols oxygenated in the terminal fragment of their side chains]. | Bioorganicheskaia khimiia 20080101 |
| Primary human astrocytes produce 24(S),25-epoxycholesterol with implications for brain cholesterol homeostasis. | Journal of neurochemistry 20071201 |
| Regulation of CYP3A4 and CYP2B6 expression by liver X receptor agonists. | Biochemical pharmacology 20071115 |
| Studies on the cholesterol-free mouse: strong activation of LXR-regulated hepatic genes when replacing cholesterol with desmosterol. | Arteriosclerosis, thrombosis, and vascular biology 20071001 |
| 9-cis-Retinoic acid (9cRA), a retinoid X receptor (RXR) ligand, exerts immunosuppressive effects on dendritic cells by RXR-dependent activation: inhibition of peroxisome proliferator-activated receptor gamma blocks some of the 9cRA activities, and precludes them to mature phenotype development. | Journal of immunology (Baltimore, Md. : 1950) 20070515 |
| Selective up-regulation of LXR-regulated genes ABCA1, ABCG1, and APOE in macrophages through increased endogenous synthesis of 24(S),25-epoxycholesterol. | The Journal of biological chemistry 20070223 |
| SREBP-2 positively regulates transcription of the cholesterol efflux gene, ABCA1, by generating oxysterol ligands for LXR. | The Biochemical journal 20061215 |
| Discovery and optimization of a novel series of liver X receptor-alpha agonists. | Bioorganic & medicinal chemistry letters 20060315 |
| [Synthesis of (24S)-hydroxy- and (24S)-24,25-epoxycholesterol analogues, potential agonists of nuclear LXR receptors]. | Bioorganicheskaia khimiia 20060101 |
| Adipocytic differentiation and liver x receptor pathways regulate the accumulation of triacylglycerols in human vascular smooth muscle cells. | The Journal of biological chemistry 20050204 |
| Statins inhibit synthesis of an oxysterol ligand for the liver x receptor in human macrophages with consequences for cholesterol flux. | Arteriosclerosis, thrombosis, and vascular biology 20041201 |
| 24(S),25-epoxycholesterol--a potential friend. | Arteriosclerosis, thrombosis, and vascular biology 20041201 |
| Enhanced synthesis of the oxysterol 24(S),25-epoxycholesterol in macrophages by inhibitors of 2,3-oxidosqualene:lanosterol cyclase: a novel mechanism for the attenuation of foam cell formation. | Circulation research 20031017 |
| Induction of intestinal ATP-binding cassette transporters by a phytosterol-derived liver X receptor agonist. | The Journal of biological chemistry 20030919 |
| X-ray crystal structure of the liver X receptor beta ligand binding domain: regulation by a histidine-tryptophan switch. | The Journal of biological chemistry 20030718 |
| Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines. | Journal of medicinal chemistry 20020509 |
| Oxysterol stimulation of epidermal differentiation is mediated by liver X receptor-beta in murine epidermis. | The Journal of investigative dermatology 20020101 |
| Key regulatory oxysterols in liver: analysis as delta4-3-ketone derivatives by HPLC and response to physiological perturbations. | Journal of lipid research 20010401 |
| Pharmacophore analysis of the nuclear oxysterol receptor LXRalpha. | Journal of medicinal chemistry 20010315 |
| Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. | Proceedings of the National Academy of Sciences of the United States of America 19990105 |
| Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. | The Journal of biological chemistry 19970207 |