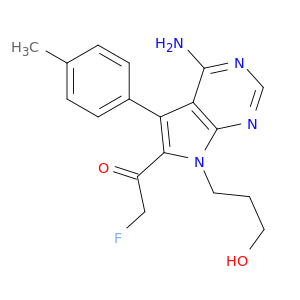
1-[4-Amino-7-(3-hydroxypropyl)-5-(4-methylphenyl)-7H-pyrrolo[2,3-d]pyrimidin-6-yl]-2-fluoroethanone
| Title | Journal |
|---|---|
| Irreversible protein kinase inhibitors: balancing the benefits and risks. | Journal of medicinal chemistry 20120726 |
| SHAFTS: a hybrid approach for 3D molecular similarity calculation. 2. Prospective case study in the discovery of diverse p90 ribosomal S6 protein kinase 2 inhibitors to suppress cell migration. | Journal of medicinal chemistry 20110526 |
| The selectivity of protein kinase inhibitors: a further update. | The Biochemical journal 20071215 |
| A clickable inhibitor reveals context-dependent autoactivation of p90 RSK. | Nature chemical biology 20070301 |
| Structural bioinformatics-based design of selective, irreversible kinase inhibitors. | Science (New York, N.Y.) 20050527 |