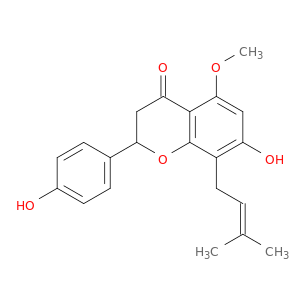
Isoxanthohumol
| Title | Journal |
|---|---|
| Inverse Virtual Screening allows the discovery of the biological activity of natural compounds. | Bioorganic & medicinal chemistry 20120601 |
| Production of 8-prenylnaringenin from isoxanthohumol through biotransformation by fungi cells. | Journal of agricultural and food chemistry 20110713 |
| Inhibitors of hyaluronan export from hops prevent osteoarthritic reactions. | Molecular nutrition & food research 20110301 |
| Quantification of xanthohumol, isoxanthohumol, 8-prenylnaringenin, and 6-prenylnaringenin in hop extracts and derived capsules using secondary standards. | Talanta 20101215 |
| Recovery and metabolism of xanthohumol in germ-free and human microbiota-associated rats. | Molecular nutrition & food research 20101001 |
| Disposition of hop prenylflavonoids in human breast tissue. | Molecular nutrition & food research 20100701 |
| In vivo estrogenic comparisons of Trifolium pratense (red clover) Humulus lupulus (hops), and the pure compounds isoxanthohumol and 8-prenylnaringenin. | Chemico-biological interactions 20081022 |
| [Main flavonoids from Sophora flavescenes]. | Yao xue xue bao = Acta pharmaceutica Sinica 20080801 |
| BACE1 inhibitory effects of lavandulyl flavanones from Sophora flavescens. | Bioorganic & medicinal chemistry 20080715 |
| Treatment of PC-3 and DU145 prostate cancer cells by prenylflavonoids from hop (Humulus lupulus L.) induces a caspase-independent form of cell death. | Phytotherapy research : PTR 20080201 |
| Microbial and dietary factors associated with the 8-prenylnaringenin producer phenotype: a dietary intervention trial with fifty healthy post-menopausal Caucasian women. | The British journal of nutrition 20071101 |
| Effect of xanthohumol and isoxanthohumol on 3T3-L1 cell apoptosis and adipogenesis. | Apoptosis : an international journal on programmed cell death 20071101 |
| Characterization of flavonoids in the extract of Sophora flavescens Ait. by high-performance liquid chromatography coupled with diode-array detector and electrospray ionization mass spectrometry. | Journal of pharmaceutical and biomedical analysis 20070903 |
| Enantioseparation of isoxanthohumol in beer by hydroxypropyl-gamma-cyclodextrin-modified micellar electrokinetic chromatography. | Journal of agricultural and food chemistry 20070808 |
| Analysis of xanthohumol and isoxanthohumol in different hop products by liquid chromatography-diode array detection-electrospray ionization tandem mass spectrometry. | Journal of chromatography. A 20070525 |
| Identification of human hepatic cytochrome P450 enzymes involved in the metabolism of 8-prenylnaringenin and isoxanthohumol from hops (Humulus lupulus L.). | Drug metabolism and disposition: the biological fate of chemicals 20060701 |
| Phytochemical-induced changes in gene expression of carcinogen-metabolizing enzymes in cultured human primary hepatocytes. | Xenobiotica; the fate of foreign compounds in biological systems 20040701 |
| Antiviral activity of hop constituents against a series of DNA and RNA viruses. | Antiviral research 20040101 |
| A binary screening assay for pro-oestrogens in food: metabolic activation using hepatic microsomes and detection with oestrogen sensitive recombinant yeast cells. | Food additives and contaminants 20021201 |