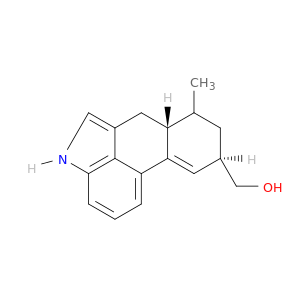
Lysergol
| Title | Journal |
|---|---|
| Simultaneous quantification of berberine and lysergol by HPLC-UV: evidence that lysergol enhances the oral bioavailability of berberine in rats. | Biomedical chromatography : BMC 20121001 |
| Quantitative determination of bioactive alkaloids lysergol and chanoclavine in Ipomoea muricata by reversed-phase high-performance liquid chromatography. | Biomedical chromatography : BMC 20120901 |
| Lysergol monohydrate. | Acta crystallographica. Section E, Structure reports online 20120201 |
| Changes in concentrations of lysergol in urine and prolactin in plasma, rectal temperature and respiration rate in sheep selected for resistance or susceptibility to ryegrass staggers and fed ergovaline. | New Zealand veterinary journal 20110901 |
| Profiling of a prescription drug library for potential renal drug-drug interactions mediated by the organic cation transporter 2. | Journal of medicinal chemistry 20110714 |
| Enantioselective total synthesis of (+)-lysergic acid, (+)-lysergol, and (+)-isolysergol by palladium-catalyzed domino cyclization of allenes bearing amino and bromoindolyl groups. | The Journal of organic chemistry 20110401 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Enantioselective synthesis of (+)-isolysergol via ring-closing metathesis. | Organic letters 20100604 |
| Large-scale separation of clavine alkaloids from Ipomoea muricata by pH-zone-refining centrifugal partition chromatography. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20090615 |
| Total synthesis of (+/-)-lysergic acid, lysergol, and isolysergol by palladium-catalyzed domino cyclization of amino allenes bearing a bromoindolyl group. | Organic letters 20081120 |
| Ergot alkaloids in Norwegian wild grasses: a mass spectrometric approach. | Rapid communications in mass spectrometry : RCM 20070101 |
| Detection of lysergic acid in ruminal fluid, urine, and in endophyte-infected tall fescue using high-performance liquid chromatography. | Journal of veterinary diagnostic investigation : official publication of the American Association of Veterinary Laboratory Diagnosticians, Inc 20060701 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Electrospray[+] tandem quadrupole mass spectrometry in the elucidation of ergot alkaloids chromatographed by HPLC: screening of grass or forage samples for novel toxic compounds. | Journal of mass spectrometry : JMS 20051101 |
| Fragmentation patterns of selected ergot alkaloids by electrospray ionization tandem quadrupole mass spectrometry. | Journal of mass spectrometry : JMS 20041101 |
| Ergot alkaloid transport across ruminant gastric tissues. | Journal of animal science 20010201 |
| Two amino acid differences in the sixth transmembrane domain are partially responsible for the pharmacological differences between the 5-HT1D beta and 5-HT1E 5-hydroxytryptamine receptors. | Journal of neurochemistry 19961101 |
| Human serotonin 1D receptor is encoded by a subfamily of two distinct genes: 5-HT1D alpha and 5-HT1D beta. | Proceedings of the National Academy of Sciences of the United States of America 19920415 |
| Substituted ergolines: potential antipsychotics with unique profile. I. Psychopharmacological characterization. | Polish journal of pharmacology and pharmacy 19880101 |