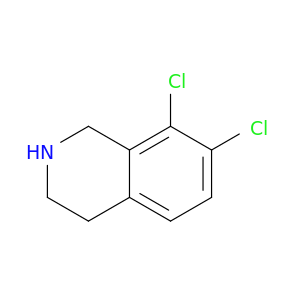
Isoquinoline, 7,9-dichloro-1,2,3,4-tetrahydro-
| Title | Journal |
|---|---|
| Synthesis of 4,5,6,7-tetrahydrothieno[3,2-c]pyridines and comparison with their isosteric 1,2,3,4-tetrahydroisoquinolines as inhibitors of phenylethanolamine N-methyltransferase. | Bioorganic & medicinal chemistry 20080101 |
| Exploring the active site of phenylethanolamine N-methyltransferase with 1,2,3,4-tetrahydrobenz[h]isoquinoline inhibitors. | Bioorganic & medicinal chemistry 20070201 |
| Comparison of the binding of 3-fluoromethyl-7-sulfonyl-1,2,3,4-tetrahydroisoquinolines with their isosteric sulfonamides to the active site of phenylethanolamine N-methyltransferase. | Journal of medicinal chemistry 20060907 |
| Structural, mutagenic, and kinetic analysis of the binding of substrates and inhibitors of human phenylethanolamine N-methyltransferase. | Journal of medicinal chemistry 20051117 |
| Exploring the active site of phenylethanolamine N-methyltransferase with 3-hydroxyethyl- and 3-hydroxypropyl-7-substituted-1,2,3,4-tetrahydroisoquinolines. | Bioorganic & medicinal chemistry letters 20050215 |
| 3-hydroxymethyl-7-(N-substituted aminosulfonyl)-1,2,3,4-tetrahydroisoquinoline inhibitors of phenylethanolamine N-methyltransferase that display remarkable potency and selectivity. | Journal of medicinal chemistry 20050113 |
| Phenylethanolamine N-methyltransferase inhibition: re-evaluation of kinetic data. | Bioorganic & medicinal chemistry letters 20040816 |
| Molecular recognition of sub-micromolar inhibitors by the epinephrine-synthesizing enzyme phenylethanolamine N-methyltransferase. | Journal of medicinal chemistry 20040101 |
| Synthesis and evaluation of 4-fluoro-8-substituted-2,3,4,5-tetrahydro-1H-2-benzazapines as selective inhibitors of phenylethanolamine N-methyltransferase versus the alpha(2)-adrenoceptor. | Journal of medicinal chemistry 20010816 |
| Phenylethanolamine N-methyltransferase kinetics: bovine versus recombinant human enzyme. | Bioorganic & medicinal chemistry letters 20010618 |
| The influence of SK & F 64139, a phenylethanolamine-N-methyltransferase inhibitor, on centrally mediated cardiovascular effects of alpha-methyldopa and clonidine. | Naunyn-Schmiedeberg's archives of pharmacology 19821201 |
| Properties of 8,9-dichloro-2,3,4,5-tetrahydro-1H-2-benzazepine, an inhibitor of norepinephrine N-methyltransferase. | Biochemical pharmacology 19810601 |