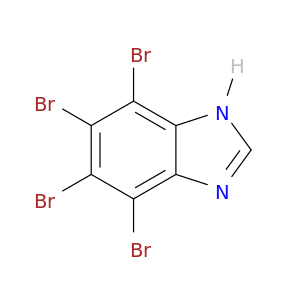
4,5,6,7-Tetrabromobenzimidazole
| Title | Journal |
|---|---|
| A subnanomolar fluorescent probe for protein kinase CK2 interaction studies. | Organic & biomolecular chemistry 20121121 |
| Modified tetrahalogenated benzimidazoles with CK2 inhibitory activity are active against human prostate cancer cells LNCaP in vitro. | Bioorganic & medicinal chemistry 20120715 |
| CK2α and CK2α' subunits differ in their sensitivity to 4,5,6,7-tetrabromo- and 4,5,6,7-tetraiodo-1H-benzimidazole derivatives. | European journal of medicinal chemistry 20120101 |
| HMGA1 is a novel downstream nuclear target of the insulin receptor signaling pathway. | Scientific reports 20120101 |
| GPCRs regulate the assembly of a multienzyme complex for purine biosynthesis. | Nature chemical biology 20111201 |
| Unbiased functional proteomics strategy for protein kinase inhibitor validation and identification of bona fide protein kinase substrates: application to identification of EEF1D as a substrate for CK2. | Journal of proteome research 20111104 |
| Design and synthesis of CK2 inhibitors. | Molecular and cellular biochemistry 20111001 |
| Protein kinase CK2 increases glutamatergic input in the hypothalamus and sympathetic vasomotor tone in hypertension. | The Journal of neuroscience : the official journal of the Society for Neuroscience 20110601 |
| Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. | Neuron 20100923 |
| Genome-wide screen in Saccharomyces cerevisiae identifies vacuolar protein sorting, autophagy, biosynthetic, and tRNA methylation genes involved in life span regulation. | PLoS genetics 20100701 |
| Anti-neoplastic effect of protein kinase CK2 inhibitor, 2-dimethylamino-4,5,6,7-tetrabromobenzimidazole (DMAT), on growth and hormonal activity of human adrenocortical carcinoma cell line (H295R) in vitro. | Cell and tissue research 20100501 |
| Dynamic regulation of a metabolic multi-enzyme complex by protein kinase CK2. | The Journal of biological chemistry 20100409 |
| Halogenated imidazole derivatives block RNA polymerase II elongation along mitogen inducible genes. | BMC molecular biology 20100101 |
| Synthesis and proapoptotic properties of new casein kinase II inhibitors. | Acta poloniae pharmaceutica 20100101 |
| Efficacy and mechanism of anti-tumor action of new potential CK2 inhibitors toward glioblastoma cells. | International journal of oncology 20091101 |
| Tetraiodobenzimidazoles are potent inhibitors of protein kinase CK2. | Bioorganic & medicinal chemistry 20091015 |
| Exploring the binding of inhibitors derived from tetrabromobenzimidazole to the CK2 protein using a QM/MM-PB/SA approach. | Journal of chemical information and modeling 20090401 |
| Synthesis of new analogs of benzotriazole, benzimidazole and phthalimide--potential inhibitors of human protein kinase CK2. | Bioorganic & medicinal chemistry 20090215 |
| The selectivity of inhibitors of protein kinase CK2: an update. | The Biochemical journal 20081101 |
| New inhibitors of protein kinase CK2, analogues of benzimidazole and benzotriazole. | Molecular and cellular biochemistry 20080901 |
| Phospho-dependent interactions between NBS1 and MDC1 mediate chromatin retention of the MRN complex at sites of DNA damage. | EMBO reports 20080801 |
| An unbiased evaluation of CK2 inhibitors by chemoproteomics: characterization of inhibitor effects on CK2 and identification of novel inhibitor targets. | Molecular & cellular proteomics : MCP 20080601 |
| Ecto-phosphorylation of CD98 regulates cell-cell interactions. | PloS one 20080101 |
| The ATP-binding site of protein kinase CK2 holds a positive electrostatic area and conserved water molecules. | Chembiochem : a European journal of chemical biology 20071015 |
| Treatment of P190 Bcr/Abl lymphoblastic leukemia cells with inhibitors of the serine/threonine kinase CK2. | Leukemia 20070101 |
| Tetrabromobenzotriazole (TBBt) and tetrabromobenzimidazole (TBBz) as selective inhibitors of protein kinase CK2: evaluation of their effects on cells and different molecular forms of human CK2. | Biochimica et biophysica acta 20051230 |
| Inspecting the structure-activity relationship of protein kinase CK2 inhibitors derived from tetrabromo-benzimidazole. | Chemistry & biology 20051101 |
| Optimization of protein kinase CK2 inhibitors derived from 4,5,6,7-tetrabromobenzimidazole. | Journal of medicinal chemistry 20041202 |
| TBBz but not TBBt discriminates between two molecular forms of CK2 in vivo and its implications. | Biochemical and biophysical research communications 20031219 |
| Polyhalogenobenzimidazoles: synthesis and their inhibitory activity against casein kinases. | Bioorganic & medicinal chemistry 20030901 |
| Alternative binding modes of an inhibitor to two different kinases. | European journal of biochemistry 20030801 |
| Selectivity of 4,5,6,7-tetrabromobenzimidazole as an ATP-competitive potent inhibitor of protein kinase CK2 from various sources. | Biochemical and biophysical research communications 20030620 |
| Sustained ER Ca2+ depletion suppresses protein synthesis and induces activation-enhanced cell death in mast cells. | The Journal of biological chemistry 20020419 |