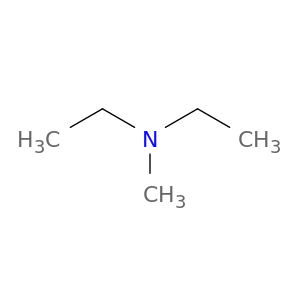
N,N-DIETHYLMETHYLAMINE
| Title | Journal |
|---|---|
| Transferable force field for equilibrium and transport properties in linear and branched monofunctional and multifunctional amines. II. Secondary and tertiary amines. | The journal of physical chemistry. B 20120531 |
| Benzophenone-based derivatives: a novel series of potent and selective dual inhibitors of acetylcholinesterase and acetylcholinesterase-induced beta-amyloid aggregation. | European journal of medicinal chemistry 20110501 |
| Nonhumidified intermediate temperature fuel cells using protic ionic liquids. | Journal of the American Chemical Society 20100721 |
| Photoreduction of oxoisoaporphines by amines: laser flash and steady-state photolysis, pulse radiolysis, and TD-DFT studies. | The journal of physical chemistry. A 20090709 |
| Characterization of poly(ethylene glycol) and PEGylated products by LC/MS with postcolumn addition of amines. | Analytical chemistry 20090115 |
| Brønsted acid-base ionic liquids for fuel cell electrolytes. | Chemical communications (Cambridge, England) 20070628 |