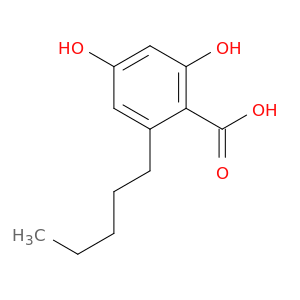
Olivetolic Acid
| Title | Journal |
|---|---|
| Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides. | Proceedings of the National Academy of Sciences of the United States of America 20120731 |
| Activities of 2,4-dihydroxy-6-n-pentylbenzoic acid derivatives. | Zeitschrift fur Naturforschung. C, Journal of biosciences 20080101 |
| Structural basis for the promiscuous biosynthetic prenylation of aromatic natural products. | Nature 20050616 |
| 6-alkylsalicylic acids and 6-alkylresorcylic acids from ants in the genus Crematogaster from Brunei. | Journal of chemical ecology 20050201 |
| Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol. | FEBS letters 19980508 |