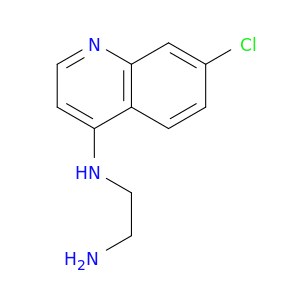
N1-(7-Chloroquinolin-4-yl)ethane-1,2-diamine
| Title | Journal |
|---|---|
| Synthesis and evaluation of hybrid drugs for a potential HIV/AIDS-malaria combination therapy. | Bioorganic & medicinal chemistry 20120901 |
| Effects of highly active novel artemisinin-chloroquinoline hybrid compounds on β-hematin formation, parasite morphology and endocytosis in Plasmodium falciparum. | Biochemical pharmacology 20110801 |
| Antiplasmodial and antitumor activity of dihydroartemisinin analogs derived via the aza-Michael addition reaction. | Bioorganic & medicinal chemistry letters 20110515 |
| Synthesis and antimalarial activities of rhenium bioorganometallics based on the 4-aminoquinoline structure. | Bioorganic & medicinal chemistry 20101115 |
| Synthesis and anti-prion activity evaluation of aminoquinoline analogues. | European journal of medicinal chemistry 20101101 |
| Design, synthesis, and in vitro antiprotozoal, antimycobacterial activities of N-{2-[(7-chloroquinolin-4-yl)amino]ethyl}ureas. | Bioorganic & medicinal chemistry 20100901 |
| Synthesis and in vitro antitubercular activity of a series of quinoline derivatives. | Bioorganic & medicinal chemistry 20090215 |
| Antimalarial dual drugs based on potent inhibitors of glutathione reductase from Plasmodium falciparum. | Journal of medicinal chemistry 20080313 |