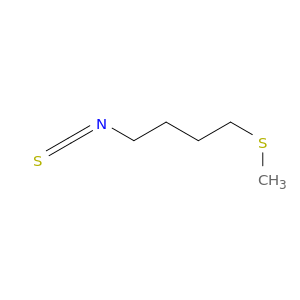
ERUCIN
| Title | Journal |
|---|---|
| Inhibition of bladder cancer by broccoli isothiocyanates sulforaphane and erucin: characterization, metabolism, and interconversion. | Molecular nutrition & food research 20121101 |
| The naturally occurring aliphatic isothiocyanates sulforaphane and erucin are weak agonists but potent non-competitive antagonists of the aryl hydrocarbon receptor. | Archives of toxicology 20121001 |
| Neuroprotective effects of erucin against 6-hydroxydopamine-induced oxidative damage in a dopaminergic-like neuroblastoma cell line. | International journal of molecular sciences 20120101 |
| Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. | Pharmacological research 20111101 |
| Comparison of isothiocyanate metabolite levels and histone deacetylase activity in human subjects consuming broccoli sprouts or broccoli supplement. | Journal of agricultural and food chemistry 20111026 |
| Induction of epoxide hydrolase and glucuronosyl transferase by isothiocyanates and intact glucosinolates in precision-cut rat liver slices: importance of side-chain substituent and chirality. | Archives of toxicology 20110801 |
| Phytochemical analysis and antimicrobial activity of Cardaria draba (L.) Desv. volatiles. | Chemistry & biodiversity 20110601 |
| Up-regulation of cytochrome P450 and phase II enzyme systems in rat precision-cut rat lung slices by the intact glucosinolates, glucoraphanin and glucoerucin. | Lung cancer (Amsterdam, Netherlands) 20110301 |
| Selective depletion of mutant p53 by cancer chemopreventive isothiocyanates and their structure-activity relationships. | Journal of medicinal chemistry 20110210 |
| Exposure to isothiocyanates suppresses urinary mutagenicity in rats treated with heterocyclic amine IQ: lack of association with CYP1 activity. | Nutrition and cancer 20110101 |
| Intact glucosinolates modulate hepatic cytochrome P450 and phase II conjugation activities and may contribute directly to the chemopreventive activity of cruciferous vegetables. | Toxicology 20101109 |
| Comparison of the apoptosis-inducing capability of sulforaphane analogues in human colon cancer cells. | Anticancer research 20100901 |
| Biological profile of erucin: a new promising anticancer agent from cruciferous vegetables. | Toxins 20100401 |
| Enhancement of arsenic trioxide cytotoxicity by dietary isothiocyanates in human leukemic cells via a reactive oxygen species-dependent mechanism. | Leukemia research 20100201 |
| Mustard oil in 'Shibori Daikon' a variety of Japanese radish, selectively inhibits the proliferation of H-ras-transformed 3Y1 cells. | Bioscience, biotechnology, and biochemistry 20091001 |
| Erucin, a new promising cancer chemopreventive agent from rocket salads, shows anti-proliferative activity on human lung carcinoma A549 cells. | Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 20090701 |
| The aliphatic isothiocyanates erucin and sulforaphane do not effectively up-regulate NAD(P)H:quinone oxidoreductase (NQO1) in human liver compared with rat. | Molecular nutrition & food research 20090701 |
| Potency of isothiocyanates to induce luciferase reporter gene expression via the electrophile-responsive element from murine glutathione S-transferase Ya. | Toxicology in vitro : an international journal published in association with BIBRA 20090601 |
| MTBITC mediates cell cycle arrest and apoptosis induction in human HepG2 cells despite its rapid degradation kinetics in the in vitro model. | Environmental and molecular mutagenesis 20090401 |
| Modulation of rat pulmonary carcinogen-metabolising enzyme systems by the isothiocyanates erucin and sulforaphane. | Chemico-biological interactions 20090127 |
| Potential skin antiinflammatory effects of 4-methylthiobutylisothiocyanate (MTBI) isolated from rocket (Eruca sativa) seeds. | BioFactors (Oxford, England) 20090101 |
| Up-regulation of the CYP1 family in rat and human liver by the aliphatic isothiocyanates erucin and sulforaphane. | Toxicology 20081030 |
| Modulation of rat hepatic and pulmonary cytochromes P450 and phase II enzyme systems by erucin, an isothiocyanate structurally related to sulforaphane. | Journal of agricultural and food chemistry 20080910 |
| Antigenotoxic properties of Eruca sativa (rocket plant), erucin and erysolin in human hepatoma (HepG2) cells towards benzo(a)pyrene and their mode of action. | Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 20080701 |
| Sulforaphane and erucin increase MRP1 and MRP2 in human carcinoma cell lines. | The Journal of nutritional biochemistry 20080401 |
| Role of PI3K/Akt and MEK/ERK signaling pathways in sulforaphane- and erucin-induced phase II enzymes and MRP2 transcription, G2/M arrest and cell death in Caco-2 cells. | Biochemical pharmacology 20050601 |
| Micronucleus formation and induction of apoptosis by different isothiocyanates and a mixture of isothiocyanates in human lymphocyte cultures. | Mutation research 20050404 |
| Sulforaphane, erucin, and iberin up-regulate thioredoxin reductase 1 expression in human MCF-7 cells. | Journal of agricultural and food chemistry 20050309 |
| Isothiocyanates as novel cytotoxic and cytostatic agents: molecular pathway on human transformed and non-transformed cells. | Biochemical pharmacology 20040915 |
| The new isothiocyanate 4-(methylthio)butylisothiocyanate selectively affects cell-cycle progression and apoptosis induction of human leukemia cells. | Investigational new drugs 20040401 |
| Aroma compound analysis of Eruca sativa (Brassicaceae) SPME headspace leaf samples using GC, GC-MS, and olfactometry. | Journal of agricultural and food chemistry 20020731 |
| Monitoring of isothiocyanates emanating from Arabidopsis thaliana upon paraquat spraying. | Journal of chromatography. A 20010330 |
| A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. | Proceedings of the National Academy of Sciences of the United States of America 19920315 |