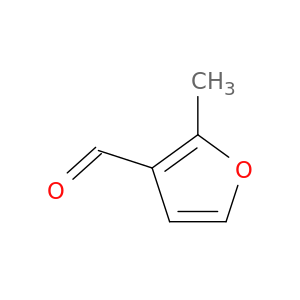
2-methyl-3-furaldehyde
| Title | Journal |
|---|---|
| Micellar electrokinetic chromatography method for the simultaneous determination of furanic compounds in honey and vegetable oils. | Talanta 20120815 |
| Regioselective synthesis and structural studies of substituted gamma-hydroxybutenolides with use of a tandem Baylis-Hillman/singlet oxygenation reaction. | The Journal of organic chemistry 20080620 |
| Addition/oxidative rearrangement of 3-furfurals and 3-furyl imines: new approaches to substituted furans and pyrroles. | Journal of the American Chemical Society 20080326 |
| Fluoride-assisted regioselective conversion of functionalized furans to alpha-substituted gamma-hydroxybutenolides using singlet oxygen. | The Journal of organic chemistry 20070803 |
| Synthesis and biological activity of novel 4,4-difluorobenzazepine derivatives as non-peptide antagonists of the arginine vasopressin V1A receptor. | Bioorganic & medicinal chemistry 20060315 |