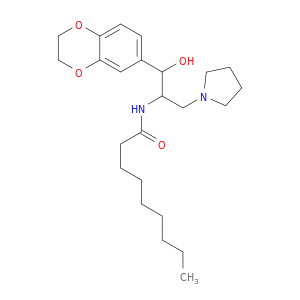
Genz-123346
| Title | Journal |
|---|---|
| A glucosylceramide synthase inhibitor protects rats against the cytotoxic effects of shiga toxin 2. | Pediatric research 20110501 |
| Glycosphingolipids and insulin resistance. | Advances in experimental medicine and biology 20110101 |
| Lipids and renal cystic disease. | Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association 20101101 |
| Reducing glycosphingolipid biosynthesis in airway cells partially ameliorates disease manifestations in a mouse model of asthma. | International immunology 20100701 |
| Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models. | Nature medicine 20100701 |
| Dual-action lipophilic iminosugar improves glycemic control in obese rodents by reduction of visceral glycosphingolipids and buffering of carbohydrate assimilation. | Journal of medicinal chemistry 20100128 |
| Inhibiting glycosphingolipid synthesis ameliorates hepatic steatosis in obese mice. | Hepatology (Baltimore, Md.) 20090701 |
| Inhibiting glycosphingolipid synthesis improves glycemic control and insulin sensitivity in animal models of type 2 diabetes. | Diabetes 20070501 |