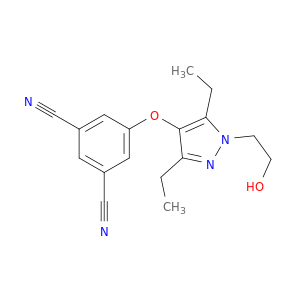
3-Cyano-5-[[3,5-diethyl-1-(2-hydroxyethyl)-1H-pyrazol-4-yl]oxy]benzonitrile
| Title | Journal |
|---|---|
| The effect of lersivirine, a next-generation NNRTI, on the pharmacokinetics of midazolam and oral contraceptives in healthy subjects. | European journal of clinical pharmacology 20121101 |
| Effect of lersivirine co-administration on pharmacokinetics of methadone in healthy volunteers. | Drug and alcohol dependence 20121101 |
| Effects of ketoconazole and valproic acid on the pharmacokinetics of the next generation NNRTI, lersivirine (UK-453,061), in healthy adult subjects. | British journal of clinical pharmacology 20120501 |
| The pharmacokinetics of lersivirine (UK-453,061) and HIV-1 protease inhibitor coadministration in healthy subjects. | Journal of acquired immune deficiency syndromes (1999) 20120501 |
| Design and synthesis of pyridone inhibitors of non-nucleoside reverse transcriptase. | Bioorganic & medicinal chemistry letters 20111215 |
| Comparison of the non-nucleoside reverse transcriptase inhibitor lersivirine with its pyrazole and imidazole isomers. | Chemical biology & drug design 20110501 |
| Nitrile-containing pharmaceuticals: efficacious roles of the nitrile pharmacophore. | Journal of medicinal chemistry 20101125 |
| Lersivirine, a nonnucleoside reverse transcriptase inhibitor with activity against drug-resistant human immunodeficiency virus type 1. | Antimicrobial agents and chemotherapy 20101001 |
| Safety and tolerability of lersivirine, a nonnucleoside reverse transcriptase inhibitor, during a 28-day, randomized, placebo-controlled, Phase I clinical study in healthy male volunteers. | Clinical therapeutics 20101001 |
| Looking for an active conformation of the future HIV type-1 non-nucleoside reverse transcriptase inhibitors. | Antiviral chemistry & chemotherapy 20100811 |
| Selective killing of human immunodeficiency virus infected cells by non-nucleoside reverse transcriptase inhibitor-induced activation of HIV protease. | Retrovirology 20100101 |
| Pyrazole NNRTIs 4: selection of UK-453,061 (lersivirine) as a development candidate. | Bioorganic & medicinal chemistry letters 20091015 |