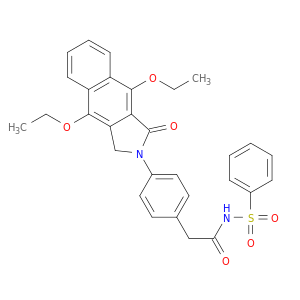
2-(4-(4,9-Diethoxy-1-oxo-1H-benzo[f]isoindol-2(3H)-yl)phenyl)-N-(phenylsulfonyl)acetamide
| Title | Journal |
|---|---|
| EP4 receptor stimulation down-regulates human eosinophil function. | Cellular and molecular life sciences : CMLS 20111101 |
| The role of the prostaglandin EP4 receptor in the regulation of human outflow facility. | Investigative ophthalmology & visual science 20110601 |
| Differential reactivity of human mammary artery and saphenous vein to prostaglandin E(2) : implication for cardiovascular grafts. | British journal of pharmacology 20110601 |
| Effect of PGE2 on DA tone by EP4 modulating Kv channels with different oxygen tension between preterm and term. | International journal of cardiology 20110217 |
| Differential regulation of the aggressive phenotype of inflammatory breast cancer cells by prostanoid receptors EP3 and EP4. | Cancer 20100601 |
| Prostaglandin E2 mediates cough via the EP3 receptor: implications for future disease therapy. | American journal of respiratory and critical care medicine 20091115 |
| EP4 and EP2 receptor subtypes involved in colonic secretion in rat. | Basic & clinical pharmacology & toxicology 20080901 |
| Activation of prostaglandin EP receptors by lubiprostone in rat and human stomach and colon. | British journal of pharmacology 20080501 |
| Molecular and pharmacological blockade of the EP4 receptor selectively inhibits both proliferation and invasion of human inflammatory breast cancer cells. | Journal of experimental therapeutics & oncology 20080101 |
| Characterization of the vasorelaxant mechanisms of the endocannabinoid anandamide in rat aorta. | British journal of pharmacology 20071101 |
| Selective PPARdelta agonist treatment increases skeletal muscle lipid metabolism without altering mitochondrial energy coupling: an in vivo magnetic resonance spectroscopy study. | American journal of physiology. Endocrinology and metabolism 20071101 |
| GW627368X ((N-{2-[4-(4,9-diethoxy-1-oxo-1,3-dihydro-2H-benzo[f]isoindol-2-yl)phenyl]acetyl} benzene sulphonamide): a novel, potent and selective prostanoid EP4 receptor antagonist. | British journal of pharmacology 20060601 |
| Piglet saphenous vein contains multiple relaxatory prostanoid receptors: evidence for EP4, EP2, DP and IP receptor subtypes. | British journal of pharmacology 20050201 |