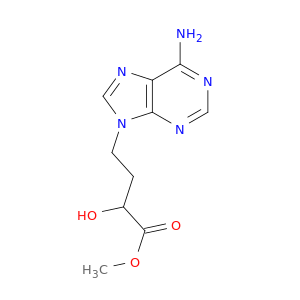
Dz2002
| Title | Journal |
|---|---|
| An endogenously anti-inflammatory role for methylation in mucosal inflammation identified through metabolite profiling. | Journal of immunology (Baltimore, Md. : 1950) 20110601 |
| AdoHcy hydrolase of Trichomonas vaginalis: studies of the effects of 5'-modified adenosine analogues and related 6-N-cyclopropyl derivatives. | Bioorganic & medicinal chemistry letters 20101215 |
| Synthesis and biological evaluation of immunosuppressive agent DZ2002 and its stereoisomers. | Bioorganic & medicinal chemistry 20081015 |
| Inhibition of transmethylation down-regulates CD4 T cell activation and curtails development of autoimmunity in a model system. | Journal of immunology (Baltimore, Md. : 1950) 20070415 |
| A reversible S-adenosyl-L-homocysteine hydrolase inhibitor ameliorates experimental autoimmune encephalomyelitis by inhibiting T cell activation. | The Journal of pharmacology and experimental therapeutics 20061101 |
| S-adenosyl-L-homocysteine hydrolase inactivation curtails ovalbumin-induced immune responses. | The Journal of pharmacology and experimental therapeutics 20060301 |