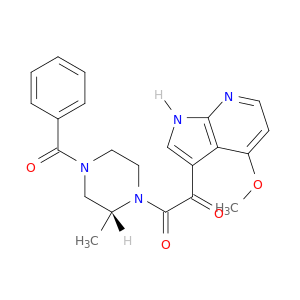
Bms-378806
| Title | Journal |
|---|---|
| Aloperine and Its Derivatives as a New Class of HIV-1 Entry Inhibitors. | ACS medicinal chemistry letters 20160310 |
| Inhibitors of HIV-1 attachment. Part 10. The discovery and structure-activity relationships of 4-azaindole cores. | Bioorganic & medicinal chemistry letters 20130101 |
| Inhibitors of HIV-1 attachment. Part 11: the discovery and structure-activity relationships associated with 4,6-diazaindole cores. | Bioorganic & medicinal chemistry letters 20130101 |
| Synthesis and biological evaluation of coumarin derivatives as inhibitors of Mycobacterium bovis (BCG). | Chemical biology & drug design 20121201 |
| An inducible cell-cell fusion system with integrated ability to measure the efficiency and specificity of HIV-1 entry inhibitors. | PloS one 20110101 |
| Development of the next generation of HIV-1 integrase inhibitors: pyrazolone as a novel inhibitor scaffold. | Bioorganic & medicinal chemistry letters 20101115 |
| Protection against HIV-envelope-induced neuronal cell destruction by HIV attachment inhibitors. | Archives of virology 20100501 |
| Inhibitors of human immunodeficiency virus type 1 (HIV-1) attachment. 5. An evolution from indole to azaindoles leading to the discovery of 1-(4-benzoylpiperazin-1-yl)-2-(4,7-dimethoxy-1H-pyrrolo[2,3-c]pyridin-3-yl)ethane-1,2-dione (BMS-488043), a drug candidate that demonstrates antiviral activity in HIV-1-infected subjects. | Journal of medicinal chemistry 20091210 |
| An antibody-recruiting small molecule that targets HIV gp120. | Journal of the American Chemical Society 20091118 |
| Heterobiaryl human immunodeficiency virus entry inhibitors. | Journal of medicinal chemistry 20090723 |
| Betulinic acid derivatives that target gp120 and inhibit multiple genetic subtypes of human immunodeficiency virus type 1. | Antimicrobial agents and chemotherapy 20080101 |
| Variation in HIV-1 R5 macrophage-tropism correlates with sensitivity to reagents that block envelope: CD4 interactions but not with sensitivity to other entry inhibitors. | Retrovirology 20080101 |
| Design and synthesis of human immunodeficiency virus entry inhibitors: sulfonamide as an isostere for the alpha-ketoamide group. | Journal of medicinal chemistry 20071227 |
| Entry inhibitor-based microbicides are active in vitro against HIV-1 isolates from multiple genetic subtypes. | Virology 20070801 |
| Quantitative determination of BMS-378806 in human plasma and urine by high-performance liquid chromatography/tandem mass spectrometry. | Journal of separation science 20070601 |
| Potent antiviral synergy between monoclonal antibody and small-molecule CCR5 inhibitors of human immunodeficiency virus type 1. | Antimicrobial agents and chemotherapy 20061001 |
| Thermodynamics of binding of a low-molecular-weight CD4 mimetic to HIV-1 gp120. | Biochemistry 20060912 |
| Prediction of the binding mode between BMS-378806 and HIV-1 gp120 by docking and molecular dynamics simulation. | Biochimica et biophysica acta 20060401 |
| Inhibition of virus entry: an antiviral mechanism of emerging prominence. | Current opinion in investigational drugs (London, England : 2000) 20060201 |
| Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. | Nature 20051103 |
| Drug firms donate compounds for anti-HIV gel. | Nature 20051103 |
| Modification and structure-activity relationship of a small molecule HIV-1 inhibitor targeting the viral envelope glycoprotein gp120. | Organic & biomolecular chemistry 20050507 |
| Small-molecule inhibitors of HIV-1 entry block receptor-induced conformational changes in the viral envelope glycoproteins. | Proceedings of the National Academy of Sciences of the United States of America 20040406 |
| Biochemical and genetic characterizations of a novel human immunodeficiency virus type 1 inhibitor that blocks gp120-CD4 interactions. | Journal of virology 20031001 |
| Discovery of 4-benzoyl-1-[(4-methoxy-1H- pyrrolo[2,3-b]pyridin-3-yl)oxoacetyl]-2- (R)-methylpiperazine (BMS-378806): a novel HIV-1 attachment inhibitor that interferes with CD4-gp120 interactions. | Journal of medicinal chemistry 20030925 |
| A small molecule HIV-1 inhibitor that targets the HIV-1 envelope and inhibits CD4 receptor binding. | Proceedings of the National Academy of Sciences of the United States of America 20030916 |