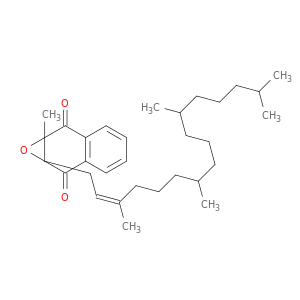
(2,3-epoxyphytyl)menaquinone
| Title | Journal |
|---|---|
| A novel mutation in VKORC1 and its effect on enzymatic activity in Japanese warfarin-resistant rats. | The Journal of veterinary medical science 20130201 |
| Functional study of the vitamin K cycle in mammalian cells. | Blood 20110310 |
| Novel insight into the mechanism of the vitamin K oxidoreductase (VKOR): electron relay through Cys43 and Cys51 reduces VKOR to allow vitamin K reduction and facilitation of vitamin K-dependent protein carboxylation. | The Journal of biological chemistry 20110304 |
| Ethnic differences in the population pharmacokinetics and pharmacodynamics of warfarin. | Journal of pharmacokinetics and pharmacodynamics 20100201 |
| A possible ethanol-catalyzed rearrangement of vitamin K(1) detected by gas chromatography/mass spectrometry. | Rapid communications in mass spectrometry : RCM 20081201 |
| Structure and function of vitamin K epoxide reductase. | Vitamins and hormones 20080101 |
| Vitamin K-dependent carboxylation. | Vitamins and hormones 20080101 |
| The conversion of vitamin K epoxide to vitamin K quinone and vitamin K quinone to vitamin K hydroquinone uses the same active site cysteines. | Biochemistry 20070619 |
| Disulfide-dependent protein folding is linked to operation of the vitamin K cycle in the endoplasmic reticulum. A protein disulfide isomerase-VKORC1 redox enzyme complex appears to be responsible for vitamin K1 2,3-epoxide reduction. | The Journal of biological chemistry 20070126 |
| Purified vitamin K epoxide reductase alone is sufficient for conversion of vitamin K epoxide to vitamin K and vitamin K to vitamin KH2. | Proceedings of the National Academy of Sciences of the United States of America 20061219 |
| Site-directed mutagenesis of coumarin-type anticoagulant-sensitive VKORC1: evidence that highly conserved amino acids define structural requirements for enzymatic activity and inhibition by warfarin. | Thrombosis and haemostasis 20051001 |
| Species comparison of vitamin K1 2,3-epoxide reductase activity in vitro: kinetics and warfarin inhibition. | Toxicology 20030801 |
| Homozygosity mapping of a second gene locus for hereditary combined deficiency of vitamin K-dependent clotting factors to the centromeric region of chromosome 16. | Blood 20021101 |
| Characterization and purification of the vitamin K1 2,3 epoxide reductases system from rat liver. | The Journal of pharmacy and pharmacology 20010401 |
| The association of vitamin K status with warfarin sensitivity at the onset of treatment. | British journal of haematology 20010301 |
| Mechanism of the abnormal vitamin K-dependent gamma-carboxylation process in human hepatocellular carcinomas. | Cancer 19940901 |
| Patulin biosynthesis: epoxidation of toluquinol and gentisyl alcohol by particulate preparations from Penicillium patulum. | Biochemistry 19891114 |