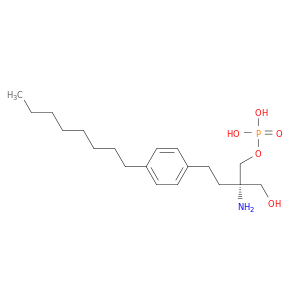
(S) FTY720 PHOSPHATE
| Title | Journal |
|---|---|
| Elevated Nuclear and Cytoplasmic FTY720-Phosphate in Mouse Embryonic Fibroblasts Suggests the Potential for Multiple Mechanisms in FTY720-Induced Neural Tube Defects. | Toxicological sciences : an official journal of the Society of Toxicology 20160301 |
| Discovery of Tetrahydropyrazolopyridine as Sphingosine 1-Phosphate Receptor 3 (S1P3)-Sparing S1P1 Agonists Active at Low Oral Doses. | Journal of medicinal chemistry 20160211 |
| FTY720 Phosphate Activates Sphingosine-1-Phosphate Receptor 2 and Selectively Couples to Gα12/13/Rho/ROCK to Induce Myofibroblast Contraction. | Molecular pharmacology 20150601 |
| Discovery of APD334: Design of a Clinical Stage Functional Antagonist of the Sphingosine-1-phosphate-1 Receptor. | ACS medicinal chemistry letters 20141211 |
| Sphingosine and FTY720 are potent inhibitors of the transient receptor potential melastatin 7 (TRPM7) channels. | British journal of pharmacology 20130301 |
| The sphingosine-1-phosphate receptor agonist FTY720 and its phosphorylated form affect the function of CD4+CD25+ T cells in vitro. | International journal of molecular medicine 20120701 |
| Identification of benzoxazole analogs as novel, S1P(3) sparing S1P(1) agonists. | Bioorganic & medicinal chemistry letters 20120615 |
| Synthesis and evaluation of CS-2100, a potent, orally active and S1P(3)- sparing S1P(1) agonist. | European journal of medicinal chemistry 20120501 |
| LC-MS/MS determination of FTY720 and FTY720-phosphate in murine intracellular compartments and human plasma. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20120301 |
| Fast simultaneous quantitative analysis of FTY720 and its metabolite FTY720-P in human blood by on-line solid phase extraction coupled with liquid chromatography-tandem mass spectrometry. | Journal of pharmaceutical and biomedical analysis 20120125 |
| Novel immunomodulators based on an oxazolin-2-one-4-carboxamide scaffold. | Bioorganic & medicinal chemistry letters 20120101 |
| Discovery of a brain-penetrant S1P₃-sparing direct agonist of the S1P₁ and S1P₅ receptors efficacious at low oral dose. | Journal of medicinal chemistry 20111013 |
| Kinetic analysis of autotaxin reveals substrate-specific catalytic pathways and a mechanism for lysophosphatidic acid distribution. | The Journal of biological chemistry 20110826 |
| Engagement of S1P₁-degradative mechanisms leads to vascular leak in mice. | The Journal of clinical investigation 20110601 |
| Fingolimod (FTY720): a recently approved multiple sclerosis drug based on a fungal secondary metabolite. | Journal of natural products 20110425 |
| Plasma gelsolin modulates cellular response to sphingosine 1-phosphate. | American journal of physiology. Cell physiology 20101201 |
| (S)-FTY720-vinylphosphonate, an analogue of the immunosuppressive agent FTY720, is a pan-antagonist of sphingosine 1-phosphate GPCR signaling and inhibits autotaxin activity. | Cellular signalling 20101001 |
| Stereochemistry-activity relationship of orally active tetralin S1P agonist prodrugs. | Bioorganic & medicinal chemistry letters 20100401 |
| Phosphorylation of the immunomodulator FTY720 inhibits programmed cell death of fibroblasts via the S1P3 receptor subtype and Bcl-2 activation. | Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 20100101 |
| Utilization of the Tango beta-arrestin recruitment technology for cell-based EDG receptor assay development and interrogation. | Journal of biomolecular screening 20091001 |
| Different response patterns of several ligands at the sphingosine-1-phosphate receptor subtype 3 (S1P(3)). | British journal of pharmacology 20090401 |
| Accumulation of fingolimod (FTY720) in lymphoid tissues contributes to prolonged efficacy. | The Journal of pharmacology and experimental therapeutics 20090301 |
| Synthesis and evaluation of alkoxy-phenylamides and alkoxy-phenylimidazoles as potent sphingosine-1-phosphate receptor subtype-1 agonists. | Bioorganic & medicinal chemistry letters 20090115 |
| Sphingosine 1-phosphate receptor agonism impairs skin dendritic cell migration and homing to secondary lymphoid tissue: association with prolonged allograft survival. | Transplant immunology 20081101 |
| Anticancer activity of FTY720: phosphorylated FTY720 inhibits autotaxin, a metastasis-enhancing and angiogenic lysophospholipase D. | Cancer letters 20080808 |
| Selective activation of G alpha i mediated signalling of S1P3 by FTY720-phosphate. | Cellular signalling 20080601 |
| Functional consequences of S1P receptor modulation in rat oligodendroglial lineage cells. | Glia 20071201 |
| A monoselective sphingosine-1-phosphate receptor-1 agonist prevents allograft rejection in a stringent rat heart transplantation model. | Chemistry & biology 20061101 |
| Novel immunomodulator FTY720 is phosphorylated in rats and humans to form a single stereoisomer. Identification, chemical proof, and biological characterization of the biologically active species and its enantiomer. | Journal of medicinal chemistry 20050811 |