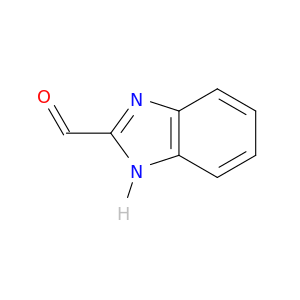
1H-Benzoimidazole-2-carboxaldehyde
| Title | Journal |
|---|---|
| Synthesis of substituted benzimidazolyl curcumin mimics and their anticancer activity. | Bioorganic & medicinal chemistry letters 20120115 |
| N-(4,6-Dimethyl-pyrimidin-2-yl)-1H-benzimidazol-2-amine. | Acta crystallographica. Section E, Structure reports online 20110301 |
| 1,4-Bis(1H-benzimidazol-2-yl)benzene methanol monosolvate. | Acta crystallographica. Section E, Structure reports online 20110101 |
| Tailored ligand acceleration of the Cu-catalyzed azide-alkyne cycloaddition reaction: practical and mechanistic implications. | Journal of the American Chemical Society 20101020 |
| Copper-catalyzed annulation of 2-formylazoles with o-aminoiodoarenes. | The Journal of organic chemistry 20100205 |
| 2-Benzimidazolyl-9-(chroman-4-yl)-purinone derivatives as JAK3 inhibitors. | Bioorganic & medicinal chemistry letters 20091201 |