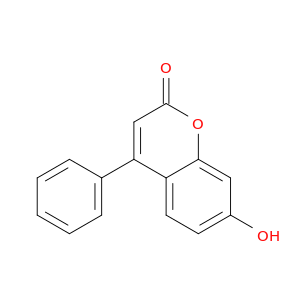
7-Hydroxy-4-phenyl-2H-chromen-2-one
| Title | Journal |
|---|---|
| Biotransformation of hydroxycoumarin derivatives by cultured suspension cells of Catharanthus roseus. | Die Pharmazie 20120501 |
| Synthesis and anti-inflammatory effects of a series of novel 7-hydroxycoumarin derivatives. | European journal of medicinal chemistry 20110901 |
| Physicochemical characterization and antioxidant activity of melanin from a novel strain of Aspergillus bridgeri ICTF-201. | Letters in applied microbiology 20110901 |
| 6,7-Dihydroxy-4-phenylcoumarin as inhibitor of aldose reductase 2. | Bioorganic & medicinal chemistry letters 20101001 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Synthesis of potential antidipsotropic isoflavones: inhibitors of the mitochondrial monoamine oxidase-aldehyde dehydrogenase pathway. | Journal of medicinal chemistry 20010927 |