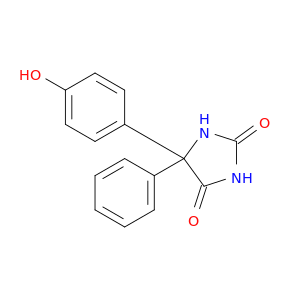
5-(4-Hydroxyphenyl)-5-phenylimidazolidine-2,4-dione
| Title | Journal |
|---|---|
| [Effect of high altitude hypoxia on the activity and protein expression of CYP2C9 and CYP2C19]. | Yao xue xue bao = Acta pharmaceutica Sinica 20120201 |
| Validated HPLC Method for Concurrent Determination of Antipyrine, Carbamazepine, Furosemide and Phenytoin and its Application in Assessment of Drug Permeability through Caco-2 Cell Monolayers. | Scientia pharmaceutica 20120101 |
| Characterization of interaction kinetics between chiral solutes and human serum albumin by using high-performance affinity chromatography and peak profiling. | Journal of chromatography. A 20110928 |
| Correlation between bilirubin glucuronidation and estradiol-3-gluronidation in the presence of model UDP-glucuronosyltransferase 1A1 substrates/inhibitors. | Drug metabolism and disposition: the biological fate of chemicals 20110201 |
| Excretion of the principal urinary metabolites of phenytoin and absolute oral bioavailability determined by use of a stable isotope in patients with epilepsy. | Therapeutic drug monitoring 20110201 |
| Phenytoin-induced gingival overgrowth: a review of the molecular, immune, and inflammatory features. | ISRN dentistry 20110101 |
| Suppression of LPS-induced matrix-metalloproteinase responses in macrophages exposed to phenytoin and its metabolite, 5-(p-hydroxyphenyl-), 5-phenylhydantoin. | Journal of inflammation (London, England) 20100101 |
| CYP2C9 amino acid residues influencing phenytoin turnover and metabolite regio- and stereochemistry. | The Journal of pharmacology and experimental therapeutics 20090601 |
| Determination of phenytoin and its major metabolite in human serum by MEKC. | Journal of chromatographic science 20090101 |
| Phenytoin metabolite renal calculus: an index case. | Journal of endourology 20080801 |
| Assessment of urinary mephenytoin metrics to phenotype for CYP2C19 and CYP2B6 activity. | European journal of clinical pharmacology 20080401 |
| Pharmacokinetics of phenytoin and its metabolite, 4'-HPPH, after intravenous and oral administration of phenytoin to diabetic rats induced by alloxan or streptozotocin. | Biopharmaceutics & drug disposition 20080101 |
| High-performance liquid chromatographic method for determination of phenytoin in rabbits receiving sildenafil. | Analytical chemistry insights 20080101 |
| Stereoselective glucuronidation of 5-(4'-hydroxyphenyl)-5-phenylhydantoin by human UDP-glucuronosyltransferase (UGT) 1A1, UGT1A9, and UGT2B15: effects of UGT-UGT interactions. | Drug metabolism and disposition: the biological fate of chemicals 20070901 |
| CYP2C9 inhibition: impact of probe selection and pharmacogenetics on in vitro inhibition profiles. | Drug metabolism and disposition: the biological fate of chemicals 20061201 |
| Paradoxical urinary phenytoin metabolite (S)/(R) ratios in CYP2C19*1/*2 patients. | Epilepsy research 20060901 |
| Studies by biointeraction chromatography of binding by phenytoin metabolites to human serum albumin. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20060519 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| Influence of the CYP2C9 AND CYP2C19 polymorphisms on phenytoin hydroxylation in healthy individuals from south India. | The Indian journal of medical research 20060501 |
| CYP2C9, CYP2C19, ABCB1 (MDR1) genetic polymorphisms and phenytoin metabolism in a Black Beninese population. | Pharmacogenetics and genomics 20051101 |
| Analytic performance evaluation of a new turbidimetric immunoassay for phenytoin on the ADVIA 1650 analyzer: effect of phenytoin metabolite and analogue. | Therapeutic drug monitoring 20050601 |
| Urinary excretion of phenytoin metabolites, 5-(4'-hydroxyphenyl)-5-phenylhydantoin and its O-glucuronide in humans and analysis of genetic polymorphisms of UDP-glucuronosyltransferases. | Drug metabolism and pharmacokinetics 20050401 |
| The effects of phenytoin and its metabolite 5-(4-hydroxyphenyl)-5-phenylhydantoin on cellular glucose transport. | Life sciences 20050304 |
| Phenytoin overview--metabolite interference in some immunoassays could be clinically important. | Archives of pathology & laboratory medicine 20040701 |
| Key structural features of ligands for activation of human pregnane X receptor. | Drug metabolism and disposition: the biological fate of chemicals 20040401 |
| The hepatic and intestinal metabolic activities of P450 in rats with surgery- and drug-induced renal dysfunction. | Pharmaceutical research 20031001 |
| Involvement of multiple UDP-glucuronosyltransferase 1A isoforms in glucuronidation of 5-(4'-hydroxyphenyl)-5-phenylhydantoin in human liver microsomes. | Drug metabolism and disposition: the biological fate of chemicals 20021101 |
| Modeling and kinetic analysis of the reaction system using whole cells with separately and co-expressed D-hydantoinase and N-carbamoylase. | Biotechnology and bioengineering 20020630 |
| Identification of catalase in human livers as a factor that enhances phenytoin dihydroxy metabolite formation by human liver microsomes. | Biochemical pharmacology 20020615 |
| Effect of albumin on phenytoin and tolbutamide metabolism in human liver microsomes: an impact more than protein binding. | Drug metabolism and disposition: the biological fate of chemicals 20020601 |
| Effects of cysteine on the pharmacokinetics of intravenous phenytoin in rats with protein-calorie malnutrition. | International journal of pharmaceutics 20011023 |
| Phenytoin metabolic ratio: a putative marker of CYP2C9 activity in vivo. | Pharmacogenetics 20011001 |
| Effect of mild therapeutic hypothermia on phenytoin pharmacokinetics. | Therapeutic drug monitoring 20010601 |
| Inhibition of phenytoin hydroxylation in human liver microsomes by several selective serotonin re-uptake inhibitors. | Epilepsy research 20010401 |
| Stereoselective determination of p-hydroxyphenyl-phenylhydantoin enantiomers in rat liver microsomal incubates by reversed-phase high-performance liquid chromatography using beta-cyclodextrin as chiral mobile phase additives. | Biomedical chromatography : BMC 20010401 |
| Stereoselective 4'-hydroxylation of phenytoin: relationship to (S)-mephenytoin polymorphism in Japanese. | British journal of clinical pharmacology 19970401 |
| Stable expression of a human liver UDP-glucuronosyltransferase (UGT2B15) with activity toward steroid and xenobiotic substrates. | Drug metabolism and disposition: the biological fate of chemicals 19940101 |