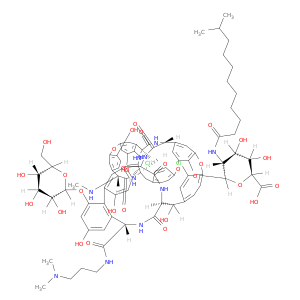
Dalbavancin
| Title | Journal |
|---|---|
| Dalbavancin (BI-387) for the treatment of complicated skin and skin structure infection. | Expert review of anti-infective therapy 20150201 |
| [In vitro activity of linezolid and dalbavancin against vancomycin-resistant enterococci]. | Mikrobiyoloji bulteni 20120701 |
| A carrier protein strategy yields the structure of dalbavancin. | Journal of the American Chemical Society 20120314 |
| MRSA new treatments on the horizon: current status. | Injury 20111201 |
| Susceptibility of molecularly characterized hospital-associated methicillin-resistant Staphylococcus aureus isolates to dalbavancin. | Journal of chemotherapy (Florence, Italy) 20111001 |
| Dalbavancin: Quantification in human plasma and urine by a new improved high performance liquid chromatography-tandem mass spectrometry method. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20110901 |
| In vitro activity of dalbavancin and telavancin against staphylococci and streptococci isolated from patients in Canadian hospitals: results of the CANWARD 2007-2009 study. | Diagnostic microbiology and infectious disease 20110301 |
| Outpatient parenteral antimicrobial therapy: Recent developments and future prospects. | Current opinion in investigational drugs (London, England : 2000) 20100801 |
| Evaluation of dalbavancin as chiral selector for HPLC and comparison with teicoplanin-based chiral stationary phases. | Chirality 20100515 |
| New lipoglycopeptides: a comparative review of dalbavancin, oritavancin and telavancin. | Drugs 20100507 |
| [Update on antimicrobial chemotherapy]. | Medecine et maladies infectieuses 20100301 |
| A comparative review of the lipoglycopeptides: oritavancin, dalbavancin, and telavancin. | Pharmacotherapy 20100101 |
| Modeled dalbavancin transmembrane clearance during intermittent and continuous renal replacement therapies. | Blood purification 20100101 |
| In vitro activity of dalbavancin against staphylococci isolated in Istanbul, Turkey. | Chemotherapy 20100101 |
| Antibiotics for gram-positive bacterial infections: vancomycin, teicoplanin, quinupristin/dalfopristin, oxazolidinones, daptomycin, dalbavancin, and telavancin. | Infectious disease clinics of North America 20091201 |
| New antimicrobial agents for methicillin-resistant Staphylococcus aureus. | Critical care and resuscitation : journal of the Australasian Academy of Critical Care Medicine 20091201 |
| Microbiology of drugs for treating multiply drug-resistant Gram-positive bacteria. | The Journal of infection 20090901 |
| Antimicrobial development in the era of emerging resistance. | Mini reviews in medicinal chemistry 20090701 |
| Multicenter evaluation of the in vitro activity of dalbavancin tested against staphylococci and streptococci in 5 European countries: results from the DECIDE Surveillance Program (2007). | Diagnostic microbiology and infectious disease 20090601 |
| Pharmacokinetics of dalbavancin in patients with renal or hepatic impairment. | Journal of clinical pharmacology 20090401 |
| A single-dose combination therapy that both prevents and treats anthrax infection. | Vaccine 20090313 |
| New antibiotics for healthcare-associated pneumonia. | Seminars in respiratory and critical care medicine 20090201 |
| In vitro activity of dalbavancin against enterococci isolates from wild animals, pets, poultry and humans in Portugal. | Journal of basic microbiology 20081201 |
| Antimicrobial agents in treatment of MRSA infections. | Disease-a-month : DM 20081201 |
| Pharmacokinetic-pharmacodynamic modeling of dalbavancin, a novel glycopeptide antibiotic. | Journal of clinical pharmacology 20080901 |
| Dalbavancin: a lipoglycopeptide antibacterial for Gram-positive infections. | Expert opinion on pharmacotherapy 20080701 |
| Dalbavancin: a new lipoglycopeptide antibiotic. | American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists 20080401 |
| Dalbavancin: a novel once-weekly lipoglycopeptide antibiotic. | Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 20080215 |
| Dalbavancin and telavancin: novel lipoglycopeptides for the treatment of Gram-positive infections. | Expert review of anti-infective therapy 20080201 |
| Review: dalbavancin--a novel lipoglycopeptide antimicrobial for gram positive pathogens. | Pakistan journal of pharmaceutical sciences 20080101 |
| Dalbavancin. | Drugs 20080101 |
| Update on prevalence and treatment of methicillin-resistant Staphylococcus aureus infections. | Expert review of anti-infective therapy 20071201 |
| Pharmacokinetics of dalbavancin in plasma and skin blister fluid. | The Journal of antimicrobial chemotherapy 20070901 |
| Dalbavancin, a long-acting lipoglycopeptide for the treatment of multidrug-resistant Gram-positive bacteria. | Expert review of anti-infective therapy 20070801 |
| Dalbavancin: a novel antimicrobial. | International journal of clinical practice 20070501 |
| Review of dalbavancin, a novel semisynthetic lipoglycopeptide. | Expert opinion on investigational drugs 20070501 |
| Dalbavancin: a review. | Drugs of today (Barcelona, Spain : 1998) 20070501 |
| Biotransformations of lipoglycopeptides to obtain novel antibiotics. | The Journal of antibiotics 20070401 |
| [Therapeutic perspectives of linezolid in the management of infections due to multiresistant Gram-positive pathogens]. | Recenti progressi in medicina 20070301 |
| Pharmacodynamics of dalbavancin studied in an in vitro pharmacokinetic system. | The Journal of antimicrobial chemotherapy 20061001 |
| Effect of dalbavancin on the normal intestinal microflora. | The Journal of antimicrobial chemotherapy 20060901 |
| Glycopeptides and glycodepsipeptides in clinical development: a comparative review of their antibacterial spectrum, pharmacokinetics and clinical efficacy. | Current opinion in investigational drugs (London, England : 2000) 20060801 |
| Understanding and manipulating glycopeptide pathways: the example of the dalbavancin precursor A40926. | Journal of industrial microbiology & biotechnology 20060701 |
| Dalbavancin: a novel lipoglycopeptide antibacterial. | Pharmacotherapy 20060701 |
| Evaluation of dalbavancin in combination with nine antimicrobial agents to detect enhanced or antagonistic interactions. | International journal of antimicrobial agents 20060601 |
| Antibacterial drug discovery and development--SRI's 11th Annual Summit. Antibacterial trends and current research. | IDrugs : the investigational drugs journal 20060601 |
| Dalbavancin: a review for dermatologists. | Dermatology online journal 20060530 |
| Dalbavancin: a new option for the treatment of gram-positive infections. | The Annals of pharmacotherapy 20060301 |
| Spectrum and potency of dalbavancin tested against 3322 Gram-positive cocci isolated in the United States Surveillance Program (2004). | Diagnostic microbiology and infectious disease 20060201 |
| Microbiologic characterization of isolates from a dalbavancin clinical trial for catheter-related bloodstream infections. | Diagnostic microbiology and infectious disease 20060201 |
| Dalbavancin activity against selected populations of antimicrobial-resistant Gram-positive pathogens. | Diagnostic microbiology and infectious disease 20051201 |
| Antimicrobial spectrum and potency of dalbavancin tested against clinical isolates from Europe and North America (2003): initial results from an international surveillance protocol. | Journal of chemotherapy (Florence, Italy) 20051201 |
| Randomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. | Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 20051115 |
| Population pharmacokinetic analysis of dalbavancin, a novel lipoglycopeptide. | Journal of clinical pharmacology 20051101 |
| New antibiotics for the treatment of severe staphylococcal infection in the critically ill patient. | Current opinion in critical care 20051001 |
| Acquisitions not a cure for anti-infectives. | Nature reviews. Drug discovery 20050901 |
| Dalbavancin compared with vancomycin for prevention of Staphylococcus aureus colonization of devices in vivo. | The Journal of infection 20050401 |
| Origin, structure, and activity in vitro and in vivo of dalbavancin. | The Journal of antimicrobial chemotherapy 20050301 |
| In vitro antistaphylococcal activity of dalbavancin, a novel glycopeptide. | The Journal of antimicrobial chemotherapy 20050301 |
| Human pharmacokinetics and rationale for once-weekly dosing of dalbavancin, a semi-synthetic glycopeptide. | The Journal of antimicrobial chemotherapy 20050301 |
| Pharmacokinetics and excretion of dalbavancin in the rat. | The Journal of antimicrobial chemotherapy 20050301 |
| Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. | Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 20050201 |
| Antimicrobial activity of dalbavancin tested against Gram-positive clinical isolates from Latin American medical centres. | Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases 20050201 |
| Dalbavancin: an investigational glycopeptide. | Expert review of anti-infective therapy 20041201 |
| Recent advances in the treatment of infections due to resistant Staphylococcus aureus. | Current opinion in infectious diseases 20041201 |
| Glycopeptides in clinical development: pharmacological profile and clinical perspectives. | Current opinion in pharmacology 20041001 |
| Activity of dalbavancin against staphylococci and streptococci, assessed by BSAC and NCCLS agar dilution methods. | The Journal of antimicrobial chemotherapy 20040901 |
| Current and new antimicrobial agents. | American heart journal 20040401 |
| Worldwide assessment of dalbavancin activity and spectrum against over 6,000 clinical isolates. | Diagnostic microbiology and infectious disease 20040201 |
| Validation of commercial dry-form broth microdilution panels and test reproducibility for susceptibility testing of dalbavancin, a new very long-acting glycopeptide. | International journal of antimicrobial agents 20040201 |
| Glycopeptide antibiotics: from conventional molecules to new derivatives. | Drugs 20040101 |
| Once-weekly dalbavancin versus standard-of-care antimicrobial regimens for treatment of skin and soft-tissue infections. | Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 20031115 |
| The gene cluster for the biosynthesis of the glycopeptide antibiotic A40926 by nonomuraea species. | Chemistry & biology 20030601 |
| Audiologic monitoring for potential ototoxicity in a phase I clinical trial of a new glycopeptide antibiotic. | Journal of the American Academy of Audiology 20030401 |
| Dalbavancin (Biosearch Italia/Versicor). | Current opinion in investigational drugs (London, England : 2000) 20020201 |