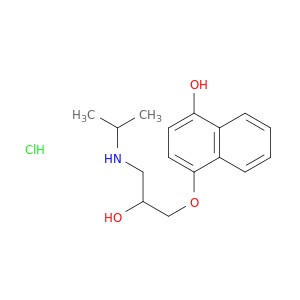
4-HYDROXY PROPRANOLOL HCL
| Title | Journal |
|---|---|
| Potential bias and mitigations when using stable isotope labeled parent drug as internal standard for LC-MS/MS quantitation of metabolites. | Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 20101201 |
| Comparative study of the oxidation of propranolol enantiomers in hepatic and small intestinal microsomes from cynomolgus and marmoset monkeys. | Chemico-biological interactions 20100105 |
| Simultaneous determination of propranolol and 4-hydroxy propranolol in human plasma by solid phase extraction and liquid chromatography/electrospray tandem mass spectrometry. | Journal of pharmaceutical and biomedical analysis 20091205 |
| Enantioselective analysis of propranolol and 4-hydroxypropranolol by CE with application to biotransformation studies employing endophytic fungi. | Electrophoresis 20091101 |
| Box-Behnken design for the optimization of an enantioselective method for the simultaneous analysis of propranolol and 4-hydroxypropranolol by CE. | Electrophoresis 20090801 |
| Involvement of SULT1A3 in elevated sulfation of 4-hydroxypropranolol in Hep G2 cells pretreated with beta-naphthoflavone. | Biochemical pharmacology 20050315 |
| Potent antioxidant properties of 4-hydroxyl-propranolol. | The Journal of pharmacology and experimental therapeutics 20040101 |
| Inactivation of rat cytochrome P450 2D enzyme by a further metabolite of 4-hydroxypropranolol, the major and active metabolite of propranolol. | Biological & pharmaceutical bulletin 20010901 |
| High-performance liquid chromatographic analysis of the sulfation of 4-hydroxypropranolol enantiomers by monkey liver cytosol. | Chirality 20010101 |