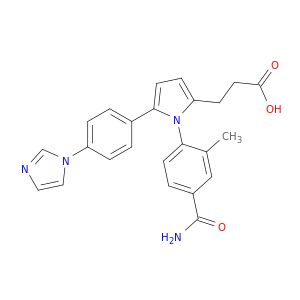
N6022
| Title | Journal |
|---|---|
| Structure-activity relationship of pyrrole based S-nitrosoglutathione reductase inhibitors: carboxamide modification. | Bioorganic & medicinal chemistry letters 20120315 |
| Mechanism of inhibition for N6022, a first-in-class drug targeting S-nitrosoglutathione reductase. | Biochemistry 20120313 |
| A nonclinical safety and pharmacokinetic evaluation of N6022: a first-in-class S-nitrosoglutathione reductase inhibitor for the treatment of asthma. | Regulatory toxicology and pharmacology : RTP 20120201 |
| Discovery of potent and novel S-nitrosoglutathione reductase inhibitors devoid of cytochrome P450 activities. | Bioorganic & medicinal chemistry letters 20111001 |
| Structure-activity relationships of pyrrole based S-nitrosoglutathione reductase inhibitors: pyrrole regioisomers and propionic acid replacement. | Bioorganic & medicinal chemistry letters 20110615 |