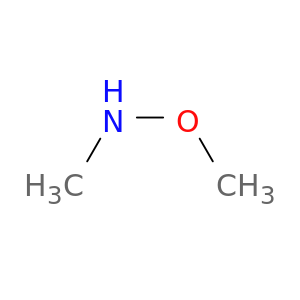
N-methoxymethylamine
| Title | Journal |
|---|---|
| Use of N,O-dimethylhydroxylamine as an anomeric protecting group in carbohydrate synthesis. | The Journal of organic chemistry 20110318 |
| Dialkylaluminum N,O-dimethylhydroxylamine complex as a reagent to mask reactive carbonyl groups in situ from nucleophiles. | Organic letters 20101203 |
| Direct one-pot synthesis of alpha-siloxy-Weinreb amides from aldehydes. | The Journal of organic chemistry 20071207 |
| 2-acetamido-N-benzyl-2-(methoxyamino)acetamides: functionalized amino acid anticonvulsants. | Acta crystallographica. Section C, Crystal structure communications 20050701 |
| A convenient method for the conversion of hindered carboxylic acids to N-methoxy-N-methyl (Weinreb) amides. | The Journal of organic chemistry 20041210 |
| Preparation of Weinreb amides using 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM). | Chemical & pharmaceutical bulletin 20040401 |