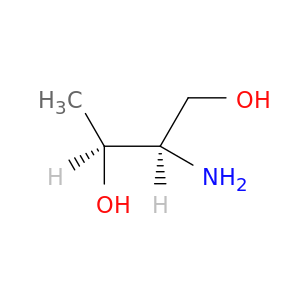
(2R,3S)-2-Aminobutane-1,3-diol
| Title | Journal |
|---|---|
| Reversed assembly of dyes in an RNA duplex compared with those in DNA. | Chemistry (Weinheim an der Bergstrasse, Germany) 20121015 |
| Bulge-like asymmetric heterodye clustering in DNA duplex results in efficient quenching of background emission based on the maximized excitonic interaction. | Chemistry (Weinheim an der Bergstrasse, Germany) 20120827 |
| Synthesis of oligonucleotides carrying thiol groups using a simple reagent derived from threoninol. | Molecules (Basel, Switzerland) 20120824 |
| Quencher-free molecular beacon tethering 7-hydroxycoumarin detects targets through protonation/deprotonation. | Bioorganic & medicinal chemistry 20120715 |
| Nick sealing by T4 DNA ligase on a modified DNA template: tethering a functional molecule on D-threoninol. | Chemistry (Weinheim an der Bergstrasse, Germany) 20110905 |
| Preparation of photoresponsive DNA tethering ortho-methylated azobenzene as a supra-photoswitch. | Current protocols in nucleic acid chemistry 20110901 |
| Synthesis and structural properties of oligonucleotides covalently linked to acridine and quindoline derivatives through a threoninol linker. | Bioorganic & medicinal chemistry 20101101 |
| Unexpectedly stable artificial duplex from flexible acyclic threoninol. | Journal of the American Chemical Society 20101027 |
| Positively charged base surrogate for highly stable 'base pairing' through electrostatic and stacking interactions. | Journal of the American Chemical Society 20090729 |
| Dye labelling of nucleosides. | Chemical biology & drug design 20090401 |
| Modulation of pK(a) of Brooker's merocyanine by DNA hybridization. | Bioconjugate chemistry 20090201 |
| In-stem molecular beacon containing a pseudo base pair of threoninol nucleotides for the removal of background emission. | Angewandte Chemie (International ed. in English) 20090101 |
| Spectroscopic labeling of A, S/T in the 1H-15N HSQC spectrum of uniformly (15N-13C) labeled proteins. | Journal of magnetic resonance (San Diego, Calif. : 1997) 20081001 |
| Threoninol as a scaffold of dyes (threoninol-nucleotide) and their stable interstrand clustering in duplexes. | Organic & biomolecular chemistry 20080821 |
| Preparation of coherent hetero clusters with threoninol scaffold. | Nucleic acids symposium series (2004) 20080101 |
| Incorporation of cationic dyes into DNA for distinct stabilization of duplex. | Nucleic acids symposium series (2004) 20080101 |