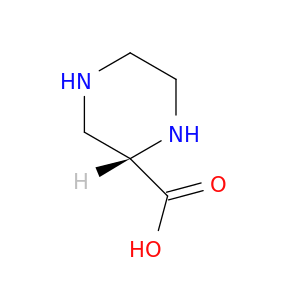
(S)-Piperazine-2-carboxylic acid
| Title | Journal |
|---|---|
| A practical synthesis of differentially protected 2-(hydroxymethyl)piperazines. | The Journal of organic chemistry 20071026 |
| S-stereoselective piperazine-2-tert-butylcarboxamide hydrolase from Pseudomonas azotoformans IAM 1603 is a novel L-amino acid amidase. | European journal of biochemistry 20040401 |
| A novel R-stereoselective amidase from Pseudomonas sp. MCI3434 acting on piperazine-2-tert-butylcarboxamide. | European journal of biochemistry 20040401 |
| Chiral synthesis and enzymatic resolution of (S)-(-)piperazine-2-carboxylic acid using enzyme alcalase. | Enantiomer 20010101 |