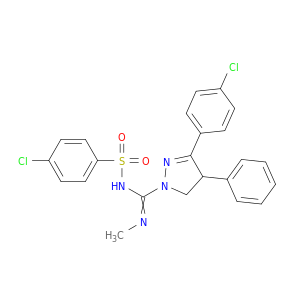
3-(4-Chlorophenyl)-N-((4-chlorophenyl)sulfonyl)-N'-methyl-4-phenyl-4,5-dihydro-1H-pyrazole-1-carboximidamide
| Title | Journal |
|---|---|
| JD-5006 and JD-5037: peripherally restricted (PR) cannabinoid-1 receptor blockers related to SLV-319 (Ibipinabant) as metabolic disorder therapeutics devoid of CNS liabilities. | Bioorganic & medicinal chemistry letters 20121001 |
| Ibipinabant attenuates β-cell loss in male Zucker diabetic fatty rats independently of its effects on body weight. | Diabetes, obesity & metabolism 20120601 |
| Surface energy analysis as a tool to probe the surface energy characteristics of micronized materials--a comparison with inverse gas chromatography. | International journal of pharmaceutics 20120117 |
| Physical stability and recrystallization kinetics of amorphous ibipinabant drug product by fourier transform raman spectroscopy. | Journal of pharmaceutical sciences 20111101 |
| The hypothermic response to bacterial lipopolysaccharide critically depends on brain CB1, but not CB2 or TRPV1, receptors. | The Journal of physiology 20110501 |
| Three-dimensional quantitative structure-selectivity relationships analysis guided rational design of a highly selective ligand for the cannabinoid receptor 2. | European journal of medicinal chemistry 20110201 |
| Pooled sample strategy in conjunction with high-resolution liquid chromatography-mass spectrometry-based background subtraction to identify toxicological markers in dogs treated with ibipinabant. | Analytical chemistry 20100501 |
| Synthesis, SAR and intramolecular hydrogen bonding pattern of 1,3,5-trisubstituted 4,5-dihydropyrazoles as potent cannabinoid CB(1) receptor antagonists. | Bioorganic & medicinal chemistry letters 20100301 |
| Bioisosteric replacement of dihydropyrazole of 4S-(-)-3-(4-chlorophenyl)-N-methyl-N'-[(4-chlorophenyl)-sulfonyl]-4-phenyl-4,5-dihydro-1H-pyrazole-1-caboxamidine (SLV-319) a potent CB1 receptor antagonist by imidazole and oxazole. | Bioorganic & medicinal chemistry letters 20080201 |
| Diaryl dihydropyrazole-3-carboxamides with significant in vivo antiobesity activity related to CB1 receptor antagonism: synthesis, biological evaluation, and molecular modeling in the homology model. | Journal of medicinal chemistry 20071129 |
| The relationship of in vivo central CB1 receptor occupancy to changes in cortical monoamine release and feeding elicited by CB1 receptor antagonists in rats. | Psychopharmacology 20060101 |
| Novel 3,4-diarylpyrazolines as potent cannabinoid CB1 receptor antagonists with lower lipophilicity. | Bioorganic & medicinal chemistry letters 20051101 |
| Synthesis, biological properties, and molecular modeling investigations of novel 3,4-diarylpyrazolines as potent and selective CB(1) cannabinoid receptor antagonists. | Journal of medicinal chemistry 20040129 |