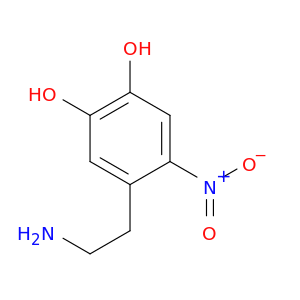
1,2-Benzenediol, 4-(2-aminoethyl)-5-nitro-
| Title | Journal |
|---|---|
| Bioinspired underwater bonding and debonding on demand. | Angewandte Chemie (International ed. in English) 20120427 |
| Sensitive electrochemical immunosensor for cancer biomarker with signal enhancement based on nitrodopamine-functionalized iron oxide nanoparticles. | Biosensors & bioelectronics 20110215 |
| Therapeutic effects of hydrogen in animal models of Parkinson's disease. | Parkinson's disease 20110101 |
| Nitrocatechols versus nitrocatecholamines as novel competitive inhibitors of neuronal nitric oxide synthase: lack of the aminoethyl side chain determines loss of tetrahydrobiopterin-antagonizing properties. | Bioorganic & medicinal chemistry letters 20020107 |
| Oxidative conversion of 6-nitrocatecholamines to nitrosating products: a possible contributory factor in nitric oxide and catecholamine neurotoxicity associated with oxidative stress and acidosis. | Chemical research in toxicology 20010901 |
| Effect of intracerebral 6-nitronoradrenaline, an endogenous catechol-O-methyltransferase (COMT) inhibitor, on striatal dopamine metabolism in anaesthetised rats. | Journal of neuroscience methods 20010815 |
| Inhibition of neuronal nitric oxide synthase by 6-nitrocatecholamines, putative reaction products of nitric oxide with catecholamines under oxidative stress conditions. | The Biochemical journal 20010515 |