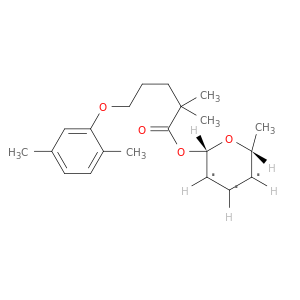
(2S,3S,4S,5R,6S)-6-((5-(2,5-Dimethylphenoxy)-2,2-dimethylpentanoyl)oxy)-3,4,5-trihydroxytetrahydro-2H-pyran-2-carboxylic acid
| Title | Journal |
|---|---|
| Mechanism-based inactivation (MBI) of cytochrome P450 enzymes: structure-activity relationships and discovery strategies to mitigate drug-drug interaction risks. | Journal of medicinal chemistry 20120614 |
| Mechanism-based inactivation of CYP2C8 by gemfibrozil occurs rapidly in humans. | Clinical pharmacology and therapeutics 20110401 |
| The CYP2C8 inhibitor gemfibrozil does not affect the pharmacokinetics of zafirlukast. | European journal of clinical pharmacology 20110201 |
| CYP2C8 activity recovers within 96 hours after gemfibrozil dosing: estimation of CYP2C8 half-life using repaglinide as an in vivo probe. | Drug metabolism and disposition: the biological fate of chemicals 20091201 |
| Construction of triple-transfected cells [organic anion-transporting polypeptide (OATP) 1B1/multidrug resistance-associated protein (MRP) 2/MRP3 and OATP1B1/MRP2/MRP4] for analysis of the sinusoidal function of MRP3 and MRP4. | Drug metabolism and disposition: the biological fate of chemicals 20091001 |
| Benzylic oxidation of gemfibrozil-1-O-beta-glucuronide by P450 2C8 leads to heme alkylation and irreversible inhibition. | Chemical research in toxicology 20090701 |
| The effect of gemfibrozil on repaglinide pharmacokinetics persists for at least 12 h after the dose: evidence for mechanism-based inhibition of CYP2C8 in vivo. | Clinical pharmacology and therapeutics 20080901 |
| Glucuronidation converts gemfibrozil to a potent, metabolism-dependent inhibitor of CYP2C8: implications for drug-drug interactions. | Drug metabolism and disposition: the biological fate of chemicals 20060101 |
| The effects of the phytoestrogenic isoflavone genistein on the hepatic disposition of preformed and hepatically generated gemfibrozil 1-O-acyl glucuronide in the isolated perfused rat liver. | The Journal of pharmacy and pharmacology 20031001 |
| Self-micellization of gemfibrozil 1-O-beta acyl glucuronide in aqueous solution. | Pharmaceutical research 20030301 |