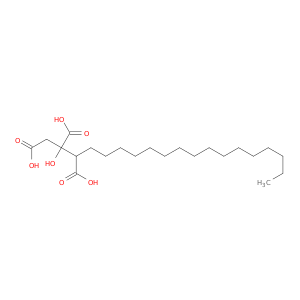
1,2,3-Nonadecanetricarboxylicacid, 2-hydroxy-
| Title | Journal |
|---|---|
| Lipid-like sulfoxides and amine oxides as inhibitors of mast cell activation. | European journal of medicinal chemistry 20110601 |
| The effect of N-ethylmaleimide on permeability transition as induced by carboxyatractyloside, agaric acid, and oleate. | Cell biochemistry and biophysics 20080101 |
| [Cellular uptake and cytotoxicity of modified chitosans as gene carriers]. | Zhongguo yi xue ke xue yuan xue bao. Acta Academiae Medicinae Sinicae 20060801 |
| Agaric acid induces mitochondrial permeability transition through its interaction with the adenine nucleotide translocase. Its dependence on membrane fluidity. | Mitochondrion 20050801 |
| Development and characterization of an immobilized enzyme reactor based on glyceraldehyde-3-phosphate dehydrogenase for on-line enzymatic studies. | Journal of chromatography. A 20030214 |