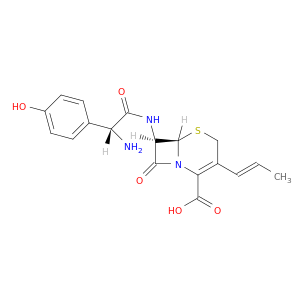
(6S,7R)-7-((R)-2-Amino-2-(4-hydroxyphenyl)acetamido)-8-oxo-3-((E)-prop-1-en-1-yl)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
| Title | Journal |
|---|---|
| Systems pharmacological analysis of drugs inducing stevens-johnson syndrome and toxic epidermal necrolysis. | Chemical research in toxicology 20150518 |
| Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps). | PLoS computational biology 20111201 |
| Acute generalized exanthematous pustulosis with severe organ dysfunction. | CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne 20090901 |
| Management of pain after tonsillectomy: a prospective, randomized clinical study. | Kulak burun bogaz ihtisas dergisi : KBB = Journal of ear, nose, and throat 20090101 |
| The stability of cefprozil in oral suspension CEFZIL. | Acta poloniae pharmaceutica 20080101 |
| Redefining the management of pediatric tonsillopharyngitis with cefprozil. | Indian journal of pediatrics 20071201 |
| Comparative effects of single-dose ceftriaxone versus three oral antibiotic regimens on stool colonization by resistant bacilli in children. | The Pediatric infectious disease journal 20070101 |
| A rare case of hepatitis associated with cefprozil therapy. | Scandinavian journal of infectious diseases 20070101 |
| Pharmacokinetics of cefprozil in plasma and middle ear fluid: in children undergoing treatment for acute otitis media. | Paediatric drugs 20070101 |
| Pharmacodynamics of cefprozil against Haemophilus influenzae in an in vitro pharmacodynamic model. | Diagnostic microbiology and infectious disease 20061201 |
| The Bla2 beta-lactamase from the live-vaccine strain of Francisella tularensis encodes a functional protein that is only active against penicillin-class beta-lactam antibiotics. | Archives of microbiology 20060901 |
| Flow-injection chemiluminescent determination of cefprozil using Tris (2,2'-bipyridyl) ruthenium (II)-permanganate system. | Journal of pharmaceutical and biomedical analysis 20060616 |
| Adverse antibiotic-induced eruptions associated with epstein barr virus infection and showing Kikuchi-Fujimoto disease-like histology. | The American Journal of dermatopathology 20060201 |
| Fatal cephalosporin-induced acute hypersensitivity myocarditis. | Pediatric cardiology 20060101 |
| A pooled analysis of seven randomized crossover studies of the palatability of cefdinir oral suspension versus amoxicillin/clavulanate potassium, cefprozil, azithromycin, and amoxicillin in children aged 4 to 8 years. | Clinical therapeutics 20051201 |
| Resistant organisms and otitis media. | The Pediatric infectious disease journal 20050901 |
| Prediction of genotoxicity of chemical compounds by statistical learning methods. | Chemical research in toxicology 20050601 |
| Cefprozil-induced rash in infectious mononucleosis. | The Annals of pharmacotherapy 20050501 |
| Effectiveness of amoxicillin, azithromycin, cefprozil and clarithromycin in the treatment of acute otitis media in children: a population-based study. | Pharmacoepidemiology and drug safety 20050301 |
| [Assessment of therapeutic effectiveness of Cefprozil in a short 5-day course of empirical antibiotic therapy in ambulatory patients with bacterial infections of the upper respiratory tract and otitis media]. | Otolaryngologia polska = The Polish otolaryngology 20050101 |
| [Cefprozil in the treatment of chronic maxillary sinusitis. Clinical and microbiological effectiveness and penetration into sinuses examination]. | Otolaryngologia polska = The Polish otolaryngology 20050101 |
| Effect of antimicrobial therapy with amoxicillin and cefprozil on bacterial interference and beta-lactamase production in the adenoids. | The Annals of otology, rhinology, and laryngology 20041101 |
| HPLC method for simultaneous determination of cefprozil diastereomers in human plasma. | Journal of pharmaceutical and biomedical analysis 20040921 |
| Pediatric tonsillopharyngitis--an evaluation of cefprozil in Indian patients. | Indian journal of pediatrics 20040701 |
| Efficacy and tolerability assessment of cefprozil in children with acute otitis media. | Indian journal of pediatrics 20040401 |
| [Resistance surveillance of common community respiratory pathogens isolated in China, 2002 - 2003]. | Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chinese journal of tuberculosis and respiratory diseases 20040301 |
| Comparison of trimethoprim-sulfamethoxazole, cephadroxil and cefprozil as prophylaxis for recurrent urinary tract infections in children. | Journal of chemotherapy (Florence, Italy) 20040201 |
| Penetration of cefprozil to middle ear effusion in children with chronic otitis media with effusion. | International journal of pediatric otorhinolaryngology 20030901 |
| Cefprozil: a review. | Indian journal of pediatrics 20030501 |
| [Multicenter, open clinical investigation on using cefprozil in therapy of otitis media in children]. | Otolaryngologia polska = The Polish otolaryngology 20030101 |
| Open-Label, parallel-group, multicenter, randomized study of cefprozil versus erythromycin in children with group A streptococcal pharyngitis/tonsillitis. | Clinical therapeutics 20011101 |
| Five days of cefprozil versus 10 days of clarithromycin in the treatment of an acute exacerbation of chronic bronchitis. | Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology 20011001 |
| Cefprozil versus high-dose amoxicillin/clavulanate in children with acute otitis media. | Clinical therapeutics 20010201 |