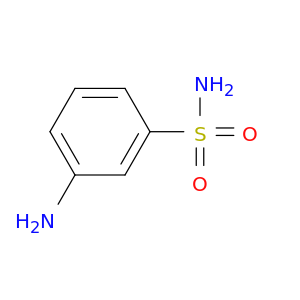
3-Aminobenzenesulfonamide
| Title | Journal |
|---|---|
| Discovery of new chromone containing sulfonamides as potent inhibitors of bovine cytosolic carbonic anhydrase. | Bioorganic & medicinal chemistry 20110601 |
| 3D-QSAR study of benzene sulfonamide analogs as carbonic anhydrase II inhibitors. | Bioorganic & medicinal chemistry letters 20100515 |
| Carbonic anhydrase inhibitors. Diazenylbenzenesulfonamides are potent and selective inhibitors of the tumor-associated isozymes IX and XII over the cytosolic isoforms I and II. | Bioorganic & medicinal chemistry 20091015 |
| Carbonic anhydrase inhibitors. Inhibition of the Rv1284 and Rv3273 beta-carbonic anhydrases from Mycobacterium tuberculosis with diazenylbenzenesulfonamides. | Bioorganic & medicinal chemistry letters 20090901 |
| Carbonic anhydrase inhibitors: Selective inhibition of the extracellular, tumor-associated isoforms IX and XII over isozymes I and II with glycosyl-thioureido-sulfonamides. | Bioorganic & medicinal chemistry letters 20070915 |
| QSAR study on topically acting sulfonamides incorporating GABA moieties: a molecular connectivity approach. | Bioorganic & medicinal chemistry letters 20060401 |
| Carbonic anhydrase inhibitors: inhibition of the human transmembrane isozyme XIV with a library of aromatic/heterocyclic sulfonamides. | Bioorganic & medicinal chemistry 20051115 |
| Carbonic anhydrase inhibitors: inhibition of the tumor-associated isozymes IX and XII with a library of aromatic and heteroaromatic sulfonamides. | Bioorganic & medicinal chemistry letters 20051101 |
| Carbonic anhydrase inhibitors: inhibition of the transmembrane isozyme XIV with sulfonamides. | Bioorganic & medicinal chemistry letters 20050901 |
| Carbonic anhydrase inhibitors. Inhibition of the membrane-bound human and bovine isozymes IV with sulfonamides. | Bioorganic & medicinal chemistry letters 20050215 |
| Carbonic anhydrase inhibitors. Inhibition of the prokariotic beta and gamma-class enzymes from Archaea with sulfonamides. | Bioorganic & medicinal chemistry letters 20041220 |
| Carbonic anhydrase inhibitors: the first QSAR study on inhibition of tumor-associated isoenzyme IX with aromatic and heterocyclic sulfonamides. | Bioorganic & medicinal chemistry letters 20040621 |
| Carbonic anhydrase inhibitors: inhibition of the tumor-associated isozyme IX with fluorine-containing sulfonamides. The first subnanomolar CA IX inhibitor discovered. | Bioorganic & medicinal chemistry letters 20040503 |
| Carbonic anhydrase inhibitors: inhibition of the tumor-associated isozyme IX with aromatic and heterocyclic sulfonamides. | Bioorganic & medicinal chemistry letters 20030324 |
| Carbonic anhydrase inhibitors. A general approach for the preparation of water-soluble sulfonamides incorporating polyamino-polycarboxylate tails and of their metal complexes possessing long-lasting, topical intraocular pressure-lowering properties. | Journal of medicinal chemistry 20020328 |
| [Reaction of 2-4-aminobenzensulfonamide structures with thymol-sodium hypochlorite]. | Die Pharmazie 20010501 |
| Carbonic anhydrase inhibitors: synthesis of sulfonamides incorporating dtpa tails and of their zinc complexes with powerful topical antiglaucoma properties. | Bioorganic & medicinal chemistry letters 20010226 |