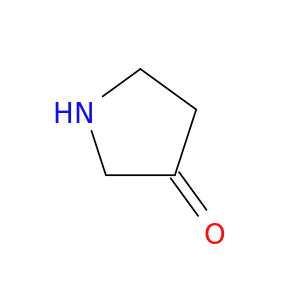
Pyrrolidin-3-one
| Title | Journal |
|---|---|
| (5E)-5-(2,4-Dichloro-benzyl-idene)-2-(piperidin-1-yl)-1,3-thia-zol-4(5H)-one. | Acta crystallographica. Section E, Structure reports online 20111101 |
| Highly active organocatalysts for asymmetric anti-Mannich reactions. | Chemistry (Weinheim an der Bergstrasse, Germany) 20110801 |
| (5E)-5-(4-Meth-oxy-benzyl-idene)-2-(piperidin-1-yl)-1,3-thia-zol-4(5H)-one. | Acta crystallographica. Section E, Structure reports online 20110801 |
| A facile synthesis and anticancer activity evaluation of spiro[thiazolidinone-isatin] conjugates. | Scientia pharmaceutica 20110101 |
| Aminodifluorosulfinium salts: selective fluorination reagents with enhanced thermal stability and ease of handling. | The Journal of organic chemistry 20100521 |
| Asymmetric synthesis of cis- and trans-2,5-disubstituted pyrrolidines from 3-oxo pyrrolidine 2-phosphonates: synthesis of (+)-preussin and analogs. | Organic letters 20080403 |
| Asymmetric hydrogenations one by one: differentiation of up to three beta-ketocarboxylic acid derivatives based on Ruthenium(II)-binap catalysis. | Chemistry (Weinheim an der Bergstrasse, Germany) 20070101 |