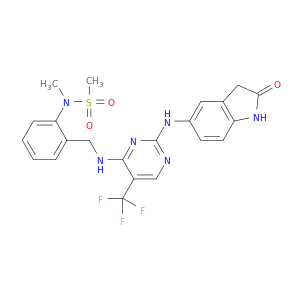
N-Methyl-N-[2-[[[2-[(2-oxo-2,3-dihydro-1H-indol-5-yl)amino]-5-trifluoromethylpyrimidin-4-yl]amino]methyl]phenyl]methanesulfonamide
| Title | Journal |
|---|---|
| Emerging targets in osteoporosis disease modification. | Journal of medicinal chemistry 20100610 |
| Sulfoximine-substituted trifluoromethylpyrimidine analogs as inhibitors of proline-rich tyrosine kinase 2 (PYK2) show reduced hERG activity. | Bioorganic & medicinal chemistry letters 20090615 |
| Structural characterization of proline-rich tyrosine kinase 2 (PYK2) reveals a unique (DFG-out) conformation and enables inhibitor design. | The Journal of biological chemistry 20090508 |