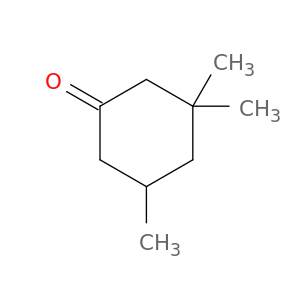
3,3,5-Trimethylcyclohexanone
| Title | Journal |
|---|---|
| Mechanistic insights into the proline-directed enantioselective heterogeneous hydrogenation of isophorone. | Langmuir : the ACS journal of surfaces and colloids 20070522 |
| 3,3,5-Trimethylcyclohexanols and derived esters: green synthetic procedures, odour evaluation and in vitro skin cytotoxicity assays. | International journal of cosmetic science 20061201 |
| Heterogeneously catalyzed asymmetric C=C hydrogenation: origin of enantioselectivity in the proline-directed Pd/isophorone system. | Journal of the American Chemical Society 20060607 |
| Biological stereoselective reduction of 3,3,5-trimethylcyclohexanone by Glomerella cingulata. | Natural product letters 20010101 |