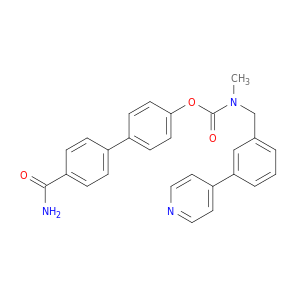
4'-Carbamoyl-[1,1'-biphenyl]-4-yl methyl(3-(pyridin-4-yl)benzyl)carbamate
| Title | Journal |
|---|---|
| WWL70 attenuates PGE(2) production derived from 2-arachidonoylglycerol in microglia by ABHD6-independent mechanism. | Journal of neuroinflammation 20170101 |
| Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. | Nature chemical biology 20090101 |
| A functional proteomic strategy to discover inhibitors for uncharacterized hydrolases. | Journal of the American Chemical Society 20070808 |