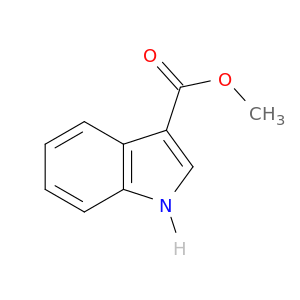
Methyl indole-3-carboxylate
| Title | Journal |
|---|---|
| Synthesis and in vitro antiproliferative activity of new 11-aminoalkylamino-substituted 5H- and 6H-indolo[2,3-b]quinolines; structure-activity relationships of neocryptolepines and 6-methyl congeners. | Bioorganic & medicinal chemistry 20120801 |
| Regioselective dibromination of methyl indole-3-carboxylate and application in the synthesis of 5,6-dibromoindoles. | Organic & biomolecular chemistry 20110721 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Enantioselective synthesis of (-)-cis-clavicipitic acid. | The Journal of organic chemistry 20071012 |