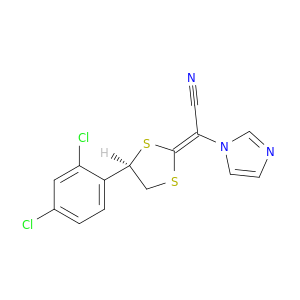
2-[(2E,4R)-4-(2,4-dichlorophenyl)-1,3-dithiolan-2-ylidene]-2-(1H-imidazol-1-yl)acetonitrile
| Title | Journal |
|---|---|
| Antimycotics suppress the Malassezia extract-induced production of CXC chemokine ligand 10 in human keratinocytes. | The Journal of dermatology 20140201 |
| Suppressive effects of antimycotics on thymic stromal lymphopoietin production in human keratinocytes. | Journal of dermatological science 20130901 |
| Childhood tinea incognito caused by Trichophyton mentagrophytes var. interdigitale mimicking pustular psoriasis. | Pediatric dermatology 20110101 |
| Efficacy of terbinafine compared to lanoconazole and luliconazole in the topical treatment of dermatophytosis in a guinea pig model. | Medical mycology 20100501 |
| In vitro antifungal activities of luliconazole, a new topical imidazole. | Medical mycology 20090101 |
| [Fungicidal activity of liranaftate against dermatophytes]. | Nihon Ishinkin Gakkai zasshi = Japanese journal of medical mycology 20090101 |
| Allergic contact dermatitis from luliconazole. | Contact dermatitis 20070501 |
| Allergic contact dermatitis from luliconazole: implication of the dithioacetal structure. | Acta dermato-venereologica 20070101 |
| [Pharmacological and clinical properties of luliconazole (Lulicon Cream 1%, Lulicon Solution 1%), a novel topical antifungal agent]. | Nihon yakurigaku zasshi. Folia pharmacologica Japonica 20060501 |
| In vitro antifungal activity of luliconazole (NND-502), a novel imidazole antifungal agent. | Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy 20040801 |
| In vitro activity of novel imidazole antifungal agent NND-502 against Malassezia species. | International journal of antimicrobial agents 20030301 |
| Achievement of complete mycological cure by topical antifungal agent NND-502 in guinea pig model of tinea pedis. | Microbiology and immunology 20030101 |
| Efficacy of NND-502, a novel imidazole antimycotic agent, in experimental models of Candida albicans and Aspergillus fumigatus infections. | International journal of antimicrobial agents 19990801 |
| In vitro and in vivo antidermatophyte activities of NND-502, a novel optically active imidazole antimycotic agent. | Antimicrobial agents and chemotherapy 19980401 |