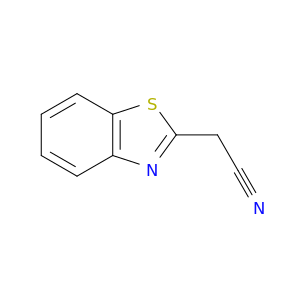
Benzothiazole-2-acetonitrile
| Title | Journal |
|---|---|
| Activation of JNK triggers release of Brd4 from mitotic chromosomes and mediates protection from drug-induced mitotic stress. | PloS one 20120101 |
| Synthesis, characterization of some benzazoles bearing pyridine moiety: search for novel anticancer agents. | European journal of medicinal chemistry 20110901 |
| Overweight worsens apoptosis, neuroinflammation and blood-brain barrier damage after hypoxic ischemia in neonatal brain through JNK hyperactivation. | Journal of neuroinflammation 20110101 |
| Solvatochromic, acid-base features and time effect of some azo dyes derived from 1,3-benzothiazol-2-ylacetonitrile: experimental and semiempirical investigations. | Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy 20100201 |
| FoxO and stress responses in the cnidarian Hydra vulgaris. | PloS one 20100101 |
| Three-dimensional quantitative structure-activity relationship (3 D-QSAR) and docking studies on (benzothiazole-2-yl) acetonitrile derivatives as c-Jun N-terminal kinase-3 (JNK3) inhibitors. | Bioorganic & medicinal chemistry letters 20061115 |
| Exploration of a binding mode of benzothiazol-2-yl acetonitrile pyrimidine core based derivatives as potent c-Jun N-terminal kinase-3 inhibitors and 3D-QSAR analyses. | Journal of chemical information and modeling 20060101 |
| Design and synthesis of the first generation of novel potent, selective, and in vivo active (benzothiazol-2-yl)acetonitrile inhibitors of the c-Jun N-terminal kinase. | Journal of medicinal chemistry 20050714 |