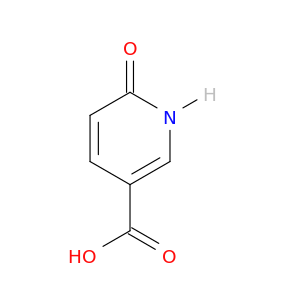
6-Oxo-1,6-dihydropyridine-3-carboxylic acid
| Title | Journal |
|---|---|
| Genomic analysis of Pseudomonas putida: genes in a genome island are crucial for nicotine degradation. | Scientific reports 20120101 |
| NAD-independent L-lactate dehydrogenase is required for L-lactate utilization in Pseudomonas stutzeri SDM. | PloS one 20120101 |
| Preparation and characterization of a molecularly imprinted polymer by grafting on silica supports: a selective sorbent for patulin toxin. | Analytical and bioanalytical chemistry 20111001 |
| A finely tuned regulatory circuit of the nicotinic acid degradation pathway in Pseudomonas putida. | Environmental microbiology 20110701 |
| Determination and confirmation of nicotinic acid and its analogues and derivates in pear and apple blossoms using high-performance liquid chromatography-diode array-electrospray ionization mass spectrometry. | Journal of agricultural and food chemistry 20091111 |
| Energetics and structure of hydroxynicotinic acids. Crystal structures of 2-, 4-, 6-hydroxynicotinic and 5-chloro-6-hydroxynicotinic acids. | The journal of physical chemistry. B 20091029 |
| Two- and three-dimensional lanthanide-organic frameworks constructed using 1-hydro-6-oxopyridine-3-carboxylate and oxalate ligands. | Dalton transactions (Cambridge, England : 2003) 20090807 |
| The Mo-Se active site of nicotinate dehydrogenase. | Proceedings of the National Academy of Sciences of the United States of America 20090707 |
| Cloning, heterologous expression, and functional characterization of the nicotinate dehydrogenase gene from Pseudomonas putida KT2440. | Biodegradation 20090701 |
| [Microplate for high throughput screening of 6-hydroxynicotinic acid transforming strains]. | Wei sheng wu xue bao = Acta microbiologica Sinica 20080101 |
| Structures with tunable strong ferromagnetic coupling: from unordered (1D) to ordered (Discrete). | Chemistry (Weinheim an der Bergstrasse, Germany) 20070101 |
| [Combination of growing culture transformation and resting cells transformation of Pseudomonas putida NA-1 for production of 6-hydroxynicotinic acid]. | Wei sheng wu xue bao = Acta microbiologica Sinica 20060201 |
| (1)H NMR spectroscopy in the diagnosis of Pseudomonas aeruginosa-induced urinary tract infection. | NMR in biomedicine 20050801 |
| [Induction of nicotinic acid hydroxylase activity of Pseudomonas putida NA-1 and optimization of transformation conditions]. | Wei sheng wu xue bao = Acta microbiologica Sinica 20050801 |
| [Screening and identification of a strain for hydroxylation of nicotinic acid]. | Wei sheng wu xue bao = Acta microbiologica Sinica 20050201 |
| Specific inhibition of a family 1A dihydroorotate dehydrogenase by benzoate pyrimidine analogues. | Journal of medicinal chemistry 20010830 |
| Elucidation of the complete Azorhizobium nicotinate catabolism pathway. | Journal of bacteriology 19921201 |