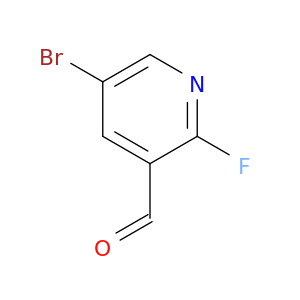
5-Bromo-2-fluoropyridine-3-carboxaldehyde
| Title | Journal |
|---|---|
| Effect of olive-mill waste addition to soil on sorption, persistence, and mobility of herbicides used in Mediterranean olive groves. | The Science of the total environment 20120701 |
| Isolation of the detoxification enzyme EgP450 from an oil palm EST library. | Pharmaceutical biology 20120101 |
| Comparative sorption and leaching study of the herbicides fluometuron and 4-chloro-2-methylphenoxyacetic acid (MCPA) in a soil amended with biochars and other sorbents. | Journal of agricultural and food chemistry 20111214 |
| Constructed wetlands as a component of the agricultural landscape: mitigation of herbicides in simulated runoff from upland drainage areas. | Chemosphere 20110601 |
| Organoclays as soil amendments to increase the efficacy and reduce the environmental impact of the herbicide fluometuron in agricultural soils. | Journal of agricultural and food chemistry 20100714 |
| Utility of Nicotiana tabacum cell suspension cultures expressing human CYP1A1, CYP1A2 and CYP3A4 to study the oxidative metabolism of the herbicide 14C-fluometuron. | Drug metabolism letters 20090101 |
| Microbial degradation of fluometuron is influenced by roundup weatherMAX. | Journal of agricultural and food chemistry 20080924 |
| The effect of vegetation on pesticide dissipation from ponded treatment wetlands: quantification using a simple model. | Chemosphere 20080701 |
| Photolysis of fluometuron in the presence of natural water constituents. | Chemosphere 20071101 |
| Degradation and sorption of fluometuron and metabolites in conservation tillage soils. | Journal of agricultural and food chemistry 20070207 |
| Photo-induced fluorescence of fluometuron in a continuous-flow multicommutation assembly. | Journal of fluorescence 20070101 |
| Accelerated solvent extraction of fluometuron from selected soils. | Journal of AOAC International 20070101 |
| Influence of watershed system management on herbicide concentrations in Mississippi Delta oxbow lakes. | The Science of the total environment 20061101 |
| Pesticide removal from cotton farm tailwater by a pilot-scale ponded wetland. | Chemosphere 20060601 |
| Combined effects of constant versus variable intensity simulated rainfall and reduced tillage management on cotton preemergence herbicide runoff. | Journal of environmental quality 20060101 |
| Laboratory assessment of atrazine and fluometuron degradation in soils from a constructed wetland. | Chemosphere 20041101 |
| Fluometuron and pendimethalin runoff from strip and conventionally tilled cotton in the southern atlantic coastal plain. | Journal of environmental quality 20040101 |
| Controlled release of the herbicide, fluometuron, from matrix granules based on fractionated organosolv lignins. | Journal of agricultural and food chemistry 20030702 |
| Fluorescent Pseudomonas isolates from Mississippi Delta oxbow lakes: in vitro herbicide biotransformations. | Environmental toxicology 20010101 |