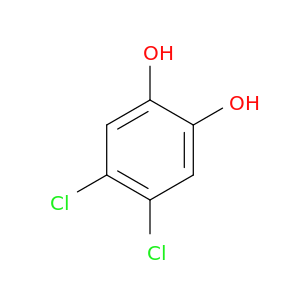
4,5-Dichlorocatechol
| Title | Journal |
|---|---|
| Characterization of the propanil biodegradation pathway in Sphingomonas sp. Y57 and cloning of the propanil hydrolase gene prpH. | Journal of hazardous materials 20111130 |
| 2,4,5-trichlororophenol and its derivatives induce biochemical and morphological changes in human peripheral blood lymphocytes in vitro. | Archives of environmental contamination and toxicology 20101101 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| Catechol 1,2-dioxygenase from the Gram-positive Rhodococcus opacus 1CP: quantitative structure/activity relationship and the crystal structures of native enzyme and catechols adducts. | Journal of structural biology 20100601 |
| Chlorophenols, chlorocatechols and chloroguaiacols induce DNA base oxidation in human lymphocytes (in vitro). | Toxicology 20100209 |
| Chlorophenols and chlorocatechols induce apoptosis in human lymphocytes (in vitro). | Toxicology letters 20091215 |
| Fine-tuning of catalytic properties of catechol 1,2-dioxygenase by active site tailoring. | Chembiochem : a European journal of chemical biology 20090417 |
| Characterization of catechol derivative removal by lignin peroxidase in aqueous mixture. | Bioresource technology 20090401 |
| Degradation of polychlorinated dibenzo-p-dioxins in aqueous solution by Fe(II)/H2O2/UV system. | Chemosphere 20060401 |
| Induction of cytotoxicity, aldehydic DNA lesions, and poly(ADP-ribose) polymerase-1 activation by catechol derivatives of pentachlorophenol in calf thymus DNA and in human breast cancer cells. | Chemical research in toxicology 20050201 |
| Chlorocatechols substituted at positions 4 and 5 are substrates of the broad-spectrum chlorocatechol 1,2-dioxygenase of Pseudomonas chlororaphis RW71. | Journal of bacteriology 20010201 |