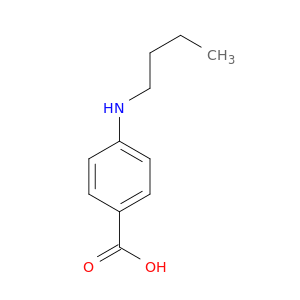
4-(Butylamino)benzoic acid
| Title | Journal |
|---|---|
| Inhibition of sensory neuronal TRPs contributes to anti-nociception by butamben. | Neuroscience letters 20120111 |
| Synthesis and SAR studies of 1,4-benzoxazine MenB inhibitors: novel antibacterial agents against Mycobacterium tuberculosis. | Bioorganic & medicinal chemistry letters 20101101 |
| The local anesthetic butamben inhibits total and L-type barium currents in PC12 cells. | Anesthesia and analgesia 20080601 |
| Microsphere-based protease assays and screening application for lethal factor and factor Xa. | Cytometry. Part A : the journal of the International Society for Analytical Cytology 20060501 |
| A novel in vitro percutaneous penetration model: evaluation of barrier properties with p-aminobenzoic acid and two of its derivatives. | Pharmaceutical research 20060501 |
| The block of total and N-type calcium conductance in mouse sensory neurons by the local anesthetic n-butyl-p-aminobenzoate. | Anesthesia and analgesia 20050601 |
| Prediction of genotoxicity of chemical compounds by statistical learning methods. | Chemical research in toxicology 20050601 |
| Kv1.1 channels of dorsal root ganglion neurons are inhibited by n-butyl-p-aminobenzoate, a promising anesthetic for the treatment of chronic pain. | The Journal of pharmacology and experimental therapeutics 20030201 |
| A sudden death following tetracaine-induced spinal anesthesia. | Legal medicine (Tokyo, Japan) 20020301 |
| Distribution of tetracaine and its metabolite in rabbits after high versus normal spinal anesthesia. | Forensic science international 20011227 |
| Blood concentrations of tetracaine and its metabolite following spinal anesthesia. | Forensic science international 20010201 |
| Simultaneous determination of mepivacaine, tetracaine, and p-butylaminobenzoic acid by high-performance liquid chromatography. | Journal of pharmacological and toxicological methods 20010101 |