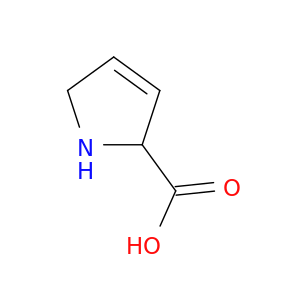
2,5-Dihydro-1h-pyrrole-2-carboxylic acid
| Title | Journal |
|---|---|
| Tailoring homochirality at surfaces: going beyond molecular handedness. | Journal of the American Chemical Society 20111012 |
| Recognition and ordering at surfaces: the importance of handedness and footedness. | Chemphyschem : a European journal of chemical physics and physical chemistry 20110606 |
| Conformational preferences and cis-trans isomerization of L-3,4-dehydroproline residue. | Biopolymers 20090101 |
| Roles of extensins in cotyledon primordium formation and shoot apical meristem activity in Nicotiana tabacum. | Journal of experimental botany 20081001 |
| Purification, characterization, and crystallization of human pyrroline-5-carboxylate reductase. | Protein expression and purification 20060901 |
| Alternative methods to limit extracellular bacterial activity for enumeration of intracellular bacteria. | Journal of microbiological methods 20060101 |
| Root border-like cells of Arabidopsis. Microscopical characterization and role in the interaction with rhizobacteria. | Plant physiology 20050601 |
| Synthesis of 4-aryl-2-pyrrolidones and beta-aryl-gamma-amino-butyric acid (GABA) analogues by Heck arylation of 3-pyrrolines with arenediazonium tetrafluoroborates. Synthesis of (+/-)-rolipram on a multigram scale and chromatographic resolution by semipreparative chiral simulated moving bed chromatography. | The Journal of organic chemistry 20050204 |
| Many amino acid substitutions in a hypoxia-inducible transcription factor (HIF)-1alpha-like peptide cause only minor changes in its hydroxylation by the HIF prolyl 4-hydroxylases: substitution of 3,4-dehydroproline or azetidine-2-carboxylic acid for the proline leads to a high rate of uncoupled 2-oxoglutarate decarboxylation. | The Journal of biological chemistry 20041231 |
| Oxidation of 3,4-dehydro-D-proline and other D-amino acid analogues by D-alanine dehydrogenase from Escherichia coli. | FEMS microbiology letters 20040915 |
| Overexpression of decorin by rat arterial smooth muscle cells enhances contraction of type I collagen in vitro. | Arteriosclerosis, thrombosis, and vascular biology 20040101 |
| Molecular characterization of NikD, a new flavoenzyme important in the biosynthesis of nikkomycin antibiotics. | Biochemistry 20021231 |
| Regulation of collagenase-3 and osteocalcin gene expression by collagen and osteopontin in differentiating MC3T3-E1 cells. | The Journal of biological chemistry 20020705 |
| Extracellular matrix regulates induction of alkaline phosphatase expression by ascorbic acid in human fibroblasts. | Journal of cellular physiology 20011101 |