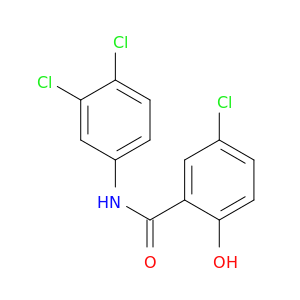
3',4',5-Trichlorosalicylanilide
| Title | Journal |
|---|---|
| New derivatives of salicylamides: Preparation and antimicrobial activity against various bacterial species. | Bioorganic & medicinal chemistry 20131101 |
| Antimycobacterial assessment of Salicylanilide benzoates including multidrug-resistant tuberculosis strains. | Molecules (Basel, Switzerland) 20121031 |
| Synthesis and in vitro antimycobacterial activity of 2-methoxybenzanilides and their thioxo analogues. | European journal of medicinal chemistry 20121001 |
| Salicylanilide derivatives block Mycobacterium tuberculosis through inhibition of isocitrate lyase and methionine aminopeptidase. | Tuberculosis (Edinburgh, Scotland) 20120901 |
| Salicylanilides: selective inhibitors of interleukin-12p40 production. | Bioorganic & medicinal chemistry 20080915 |
| Relationship between the structure and antimycobacterial activity of substituted salicylanilides. | Archiv der Pharmazie 20030301 |